1. Background
Non-alcoholic fatty liver disease (NAFLD) is among the most prevalent causes of liver disease in the world (1) and represents a spectrum of metabolic disorders that are mainly characterized as hepatic steatosis occurring in the lack of considerable alcohol consumption. The NAFLD has a spectrum of diseases, such as simple steatosis to non-alcoholic steatohepatitis (NASH), which may progress to the final stages of the disease. This disease is characterized by a high level of hepatic triglyceride storage and is mainly associated with obesity and diabetes (2). Non-alcoholic steatohepatitis, in another way, is determined by liver swelling and fibrosis and can progress to liver cirrhosis. The complex mechanisms that bring about the development of steatosis and can progress to NASH are still turbid (3). It is important to comprehend the NASH pathogenic mechanisms for implementing effective prevention and/or treatment approaches against this disease. In 1998, the two-hit theory was proposed in relation to this disease, considering fat accumulation as the first hit. Then, the incremented oxidative stress was presented as the major second hit (4). It has been recently evidenced that in NASH patients, hepatocyte apoptosis rate is high, with the enlargement of apoptosis relating to hepatic inflammation rather than simple steatosis. It is indicated that apoptosis could be included in NASH causation (5, 6). The last in vitro evidence revealed that Bcl-2 family members, such as anti-apoptotic proteins (such as Bcl-xL or Bcl-2) and pro-apoptotic proteins (like Bax) could be prime candidates for cell apoptosis c-Jun n-terminal kinase (JNK) regulation (7, 8). For instance, JNK activation can increase pro-apoptotic proteins or prevent anti-apoptotic proteins, resulting in the imbalanced Bcl-2 family. The ratio between pro- and anti-apoptotic proteins can influence or determine cell death or survival to some extent (9). By developing animal NASH models, a beneficial tool is provided to assess the potential mechanisms included in its pathogenesis (10). Recently, several nutritional animal models have been developed for high-fat diet (HFD) consumption for NASH. For instance, the pathogenic and histological features of NASH were developed by mice that received a high-fat diet for nine weeks. However, in this model, some of the biochemical changes in the liver did not imitate those found in NASH patients (11). Among the non-pharmacological achievements, physical exercise is recognized to decrease metabolic-related disorders such as NAFLD by promoting mechanisms of multisystem adaptations, promoting interaction between organs, and regulating prometabolic effects (12). The basic biological mechanisms of the effects of exercise on NAFLD liver are not well understood. In a formerly conducted study, the evidence showed that the circulating marker of hepatocyte apoptosis is reduced by endurance exercise in sedentary obese people with NAFLD, and this decrement may be associated with the capacity of fat oxidation (13). Also, findings demonstrated that endurance exercise prevented the release of cytochrome c from mitochondria and decreased potential membrane disruption. Accordingly, mitochondrial direct apoptosis signals caused NAFLD in the liver cells of rats with HFD (14). Nevertheless, the main mechanism and role of exercise training in the apoptosis of liver cells are still not fully understood.
2. Objectives
The current study aimed to investigate the effect of two exercise protocols with and without caloric restriction on apoptosis and liver damage in rats with NAFLD.
3. Methods
3.1. Animals
In the current study, 80 eight-week-old male Wistar rats were purchased from the Pasteur Institute of Iran. After transferring to the laboratory environment, they possessed free access to food and water for 2 weeks to adapt to the laboratory environment. The experimental rats were housed in translucent polycarbonate cages and in a temperature environment of 22 ± 3°C and humidity of 40 to 60% and were kept under light and darkness cycle 12:12. Based on the directions of the Ethics Committee for working with laboratory animals and with the code accepted by the Research Ethics Committee under the number IR.SSRC.REC.1402.035. Any physical abuse and unnecessary methods were prevented in different phases of the work, and rats’ weights were measured once a week on a definite day. To induce fatty liver at the end of the second week, 64 rats were randomly subjected to a high-fat diet (19% protein, 47% carbohydrate, and 34% fat) for 8 weeks (15). Another 16 rats were placed as a sham group in the control group that received normal food. In the following, 64 rats were randomly classified into 8 groups after receiving a high-fat diet for 8 weeks: Higher fat control (HFC), sham, caloric restriction (CR), caloric restriction + aerobic exercise (CA), and aerobic exercise. The calorie restriction group received 60% of the daily diet.
3.2. Exercise Protocol
The training groups performed the training program for 8 and 12 weeks and 5 sessions per week. The activity relative intensity during the exercise training program was maintained at 24 - 33 meters per minute with a slope of 15%. The duration of training started from 10 minutes in the first week and reached 60 minutes in the fifth week, and it continued until the twelfth week (16).
3.3. Sampling
Followed by the last training session (48 h) (fasting for 12 - 14 hours) at the end of the eighth and twelfth week, intraperitoneal injection of 60 and 50 mg of ketamine and xylazine per kilogram of body weight was considered for anesthetizing the rats, and underwent surgery (17). The tissue sample extracted from the lower lobe of the liver was frozen immediately at -80°C, and the hematoxylin-eosin (H&E) staining was used to check the amount of tissue damage. Also, the method of real-time PCR method was utilized for measuring the expression of Bax and Bcl-2 genes.
3.4. Real-time PCR Analysis
First, the liver tissue samples were homogenized, and then 1 mL of Trizol was added for 50 to 100 mg of tissue to achieve cell lysis. Next, for 1 mL of Trizol added in the previous step, 200 microliters of chloroform were added to the samples, then the microtubes were incubated at room temperature for 15 minutes and centrifuged at 4°C and 12,000 rpm for 15 minutes. The clear phase containing RNA was separated and transferred to another microtube, and isopropanol was added to it. Then, they were centrifuged for 10 minutes, and the remaining precipitate was washed with 75% ethanol, and the remaining materials were centrifuged again for 5 minutes, the supernatant phase was drained, and the microtubes were placed upside down on a sterile napkin for 15 minutes to semi-dry the sediment and evaporate the alcohol. After half-drying the sediment, 20 - 30 microliters of DEPC water were added to each microtube to dissolve the sediment. To prepare the RT mix for making cDNA, RT buffer, RT enzyme, oligo dT primer, and DEPC water are mixed together and then distributed in 9 microliter volumes in 2.0 mL microtubes. Prepared microtubes containing RT mix and RNA samples were placed in a thermocycler or heater block dry, and cDNA samples were prepared. Next, 9 microliters of PCR mixture were added for each microliter of cDNA, and the microtube ready for reaction was placed in the real-time PCR machine after mixing. After the end of the experiment, the numbers related to the reference gene and the main gene of each sample were put in the 2-ΔΔCT formula, and the relative changes of each gene were calculated (Table 1).
| Gene Name | Primer Sequence |
|---|---|
| Bcl-2 | |
| Forward | GAATCAAGTGTTCGTCATAAC |
| Reverse | GTTATCATACCCTGTTCTCC |
| Bax | |
| Forward | TTGCTACAGGGTTTCATCCAGG |
| Reverse | CACTCGCTCAGCTTCTTGGT |
Characteristics of the Primers used in the Real-time PCR
3.5. H&E Analysis
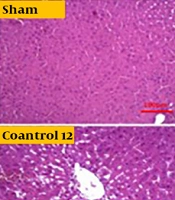
First, the samples were placed in the incubator at a temperature of 90°C for 20 minutes to melt the paraffin of the samples. In the next step of deparaffinization, the samples were placed into Xylol 1 and 2 (Sigma-1330-20-7) for 15 minutes. Afterward, the samples were placed in hematoxylin dye (Sigma-H9627) for 7 seconds; then, they were washed in distilled water for 1 minute. Subsequently, the samples were placed in lithium carbonate (Sigma 05680.1) for 2 seconds, and Eosin (HT110116-Sigma) was added for 3 minutes. The slides were exposed to absolute alcohol in order to dehydrate the tissue. Finally, a drop of ethanol was added to the samples, and the samples were photographed with a light microscope (LABOMED).
3.6. Statistical Methods
Shapiro-Wilk test was used to check the normal distribution of the data, and the Leven test was used to determine the variance or uniform distribution of the groups. Independent T-test was used to compare the high-fat and sham diet groups. Two-way ANOVA analysis of variance test at the significance level of P < 0.05 and Tukey's post hoc test were used to compare variables between groups. All statistical analyses and graphs were done with GraphPad Prism 9.5 software.
4. Results
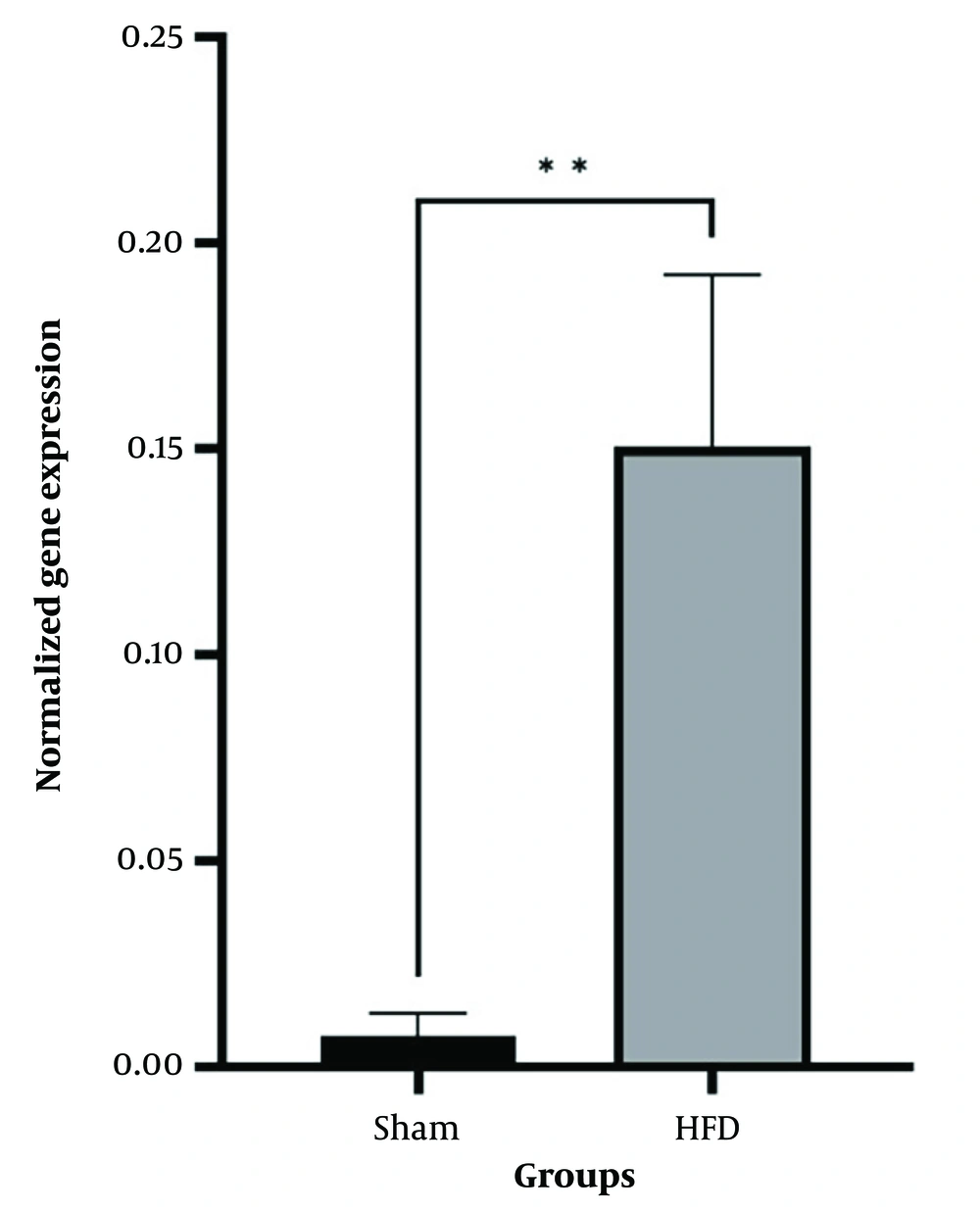
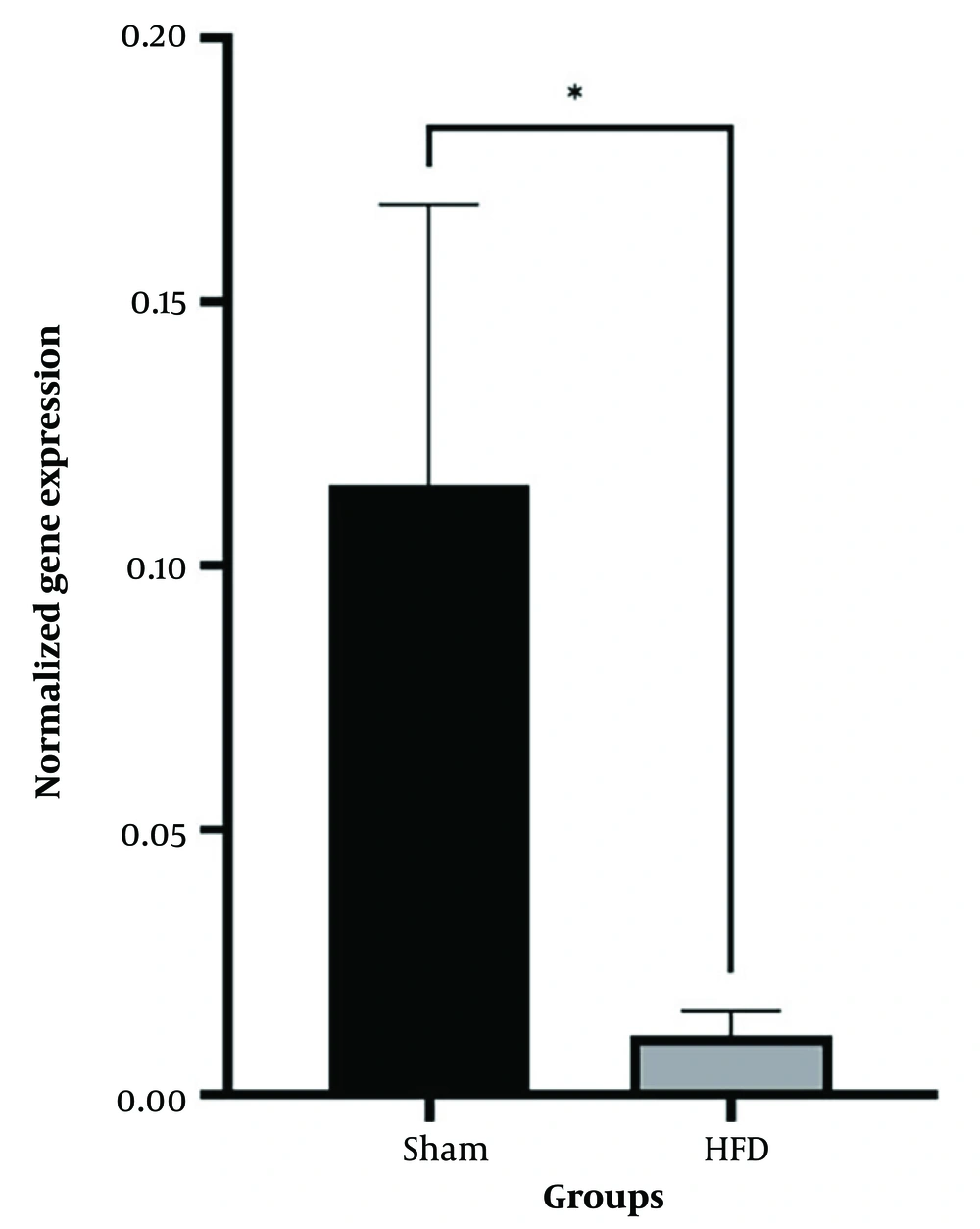
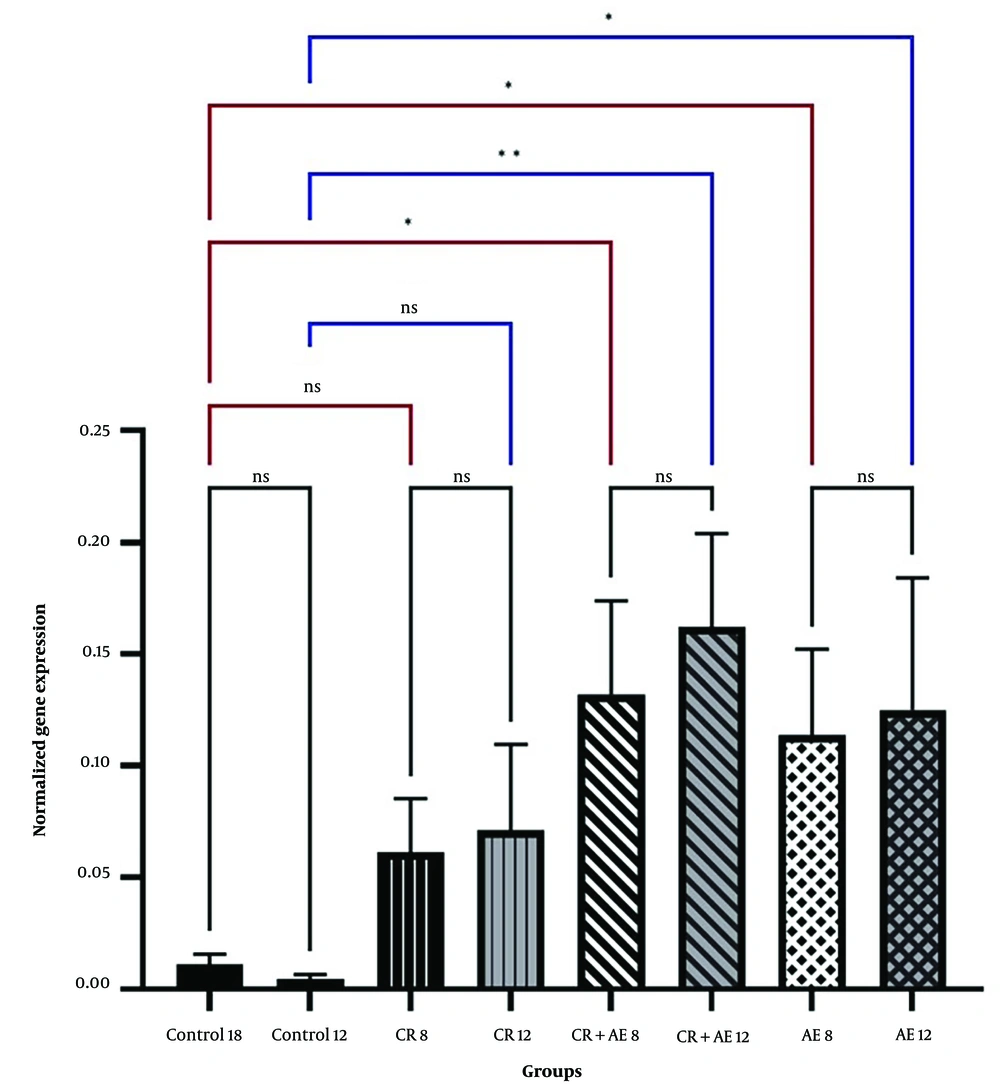
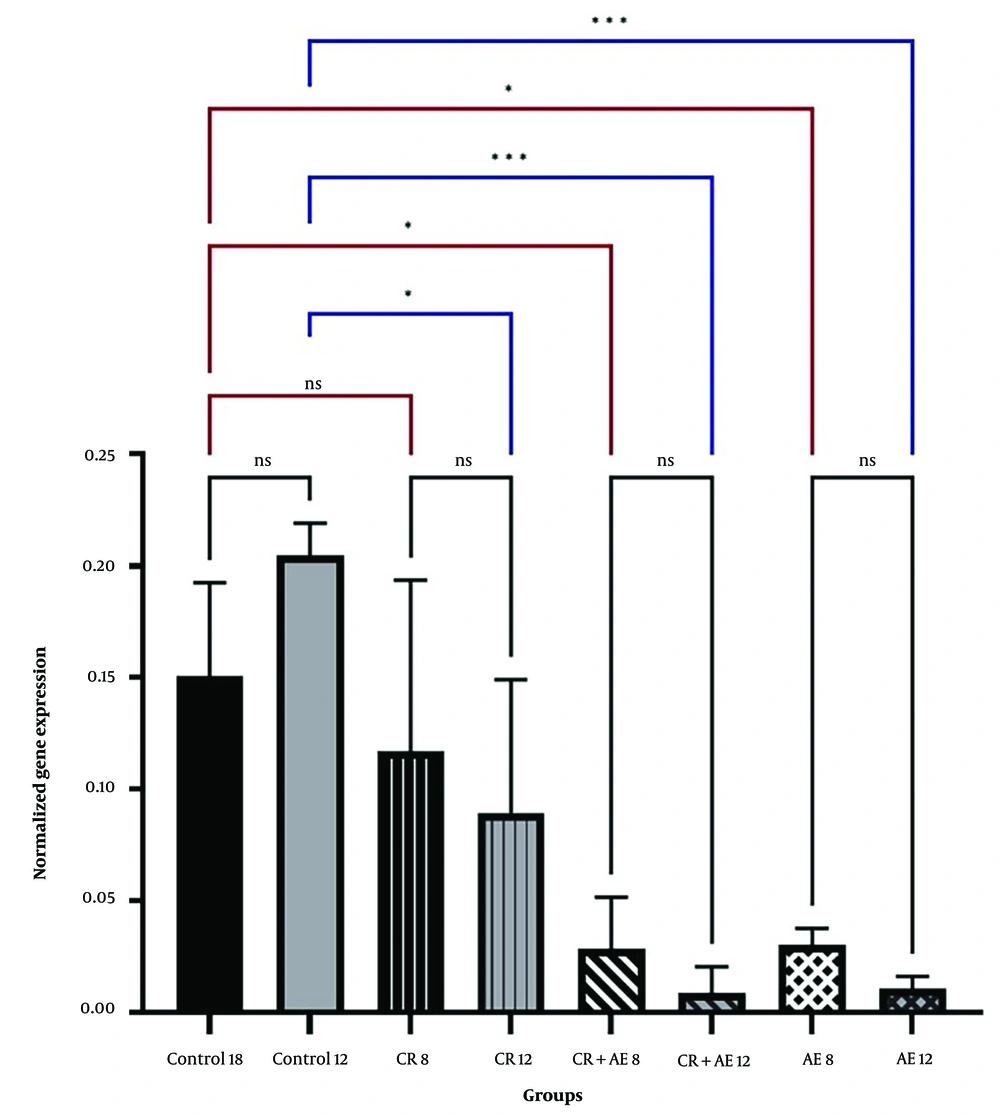
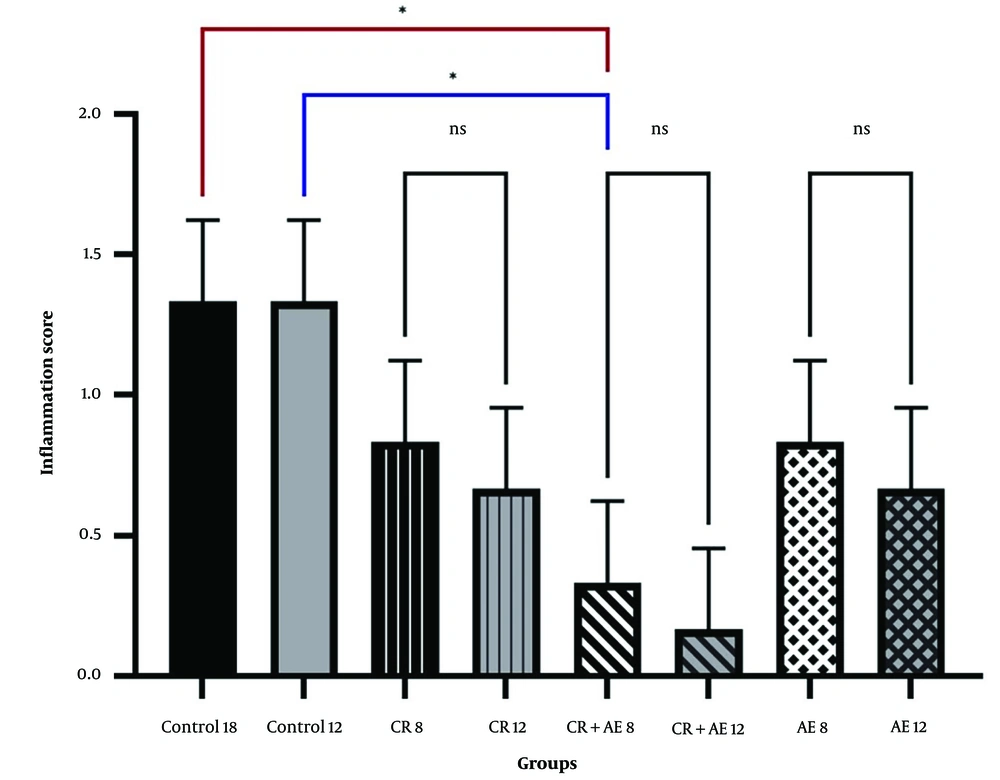
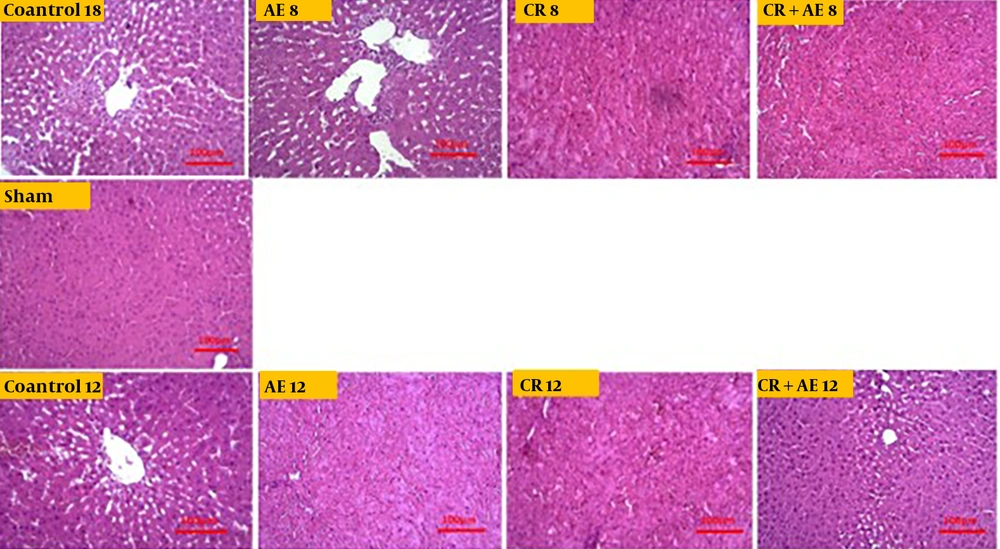
Independent T-test between high-fat and sham groups showed that 8 weeks of a high-fat diet caused a considerable difference in Bax and Bcl-2 levels (P < 0.05). Therefore, the group that received a high-fat diet for 8 weeks was referred to as the control group (Figures 1 and 2). The findings showed that Bcl-2 gene expression considerably increased at 8 and 12 weeks in the CR+ aerobic exercise (AE) and AE groups compared to the control group (P < 0.05). No considerable difference was observed between the CR, CR+AE, and AE groups in both protocols. Also, the comparison between the 8- and 12-week protocols showed that the expression of Bcl-2 increased in the CR12, CR+AE12, and AE12 groups in the 12-week protocol compared to the 8-week groups, which was not significant (P > 0.05) (Figure 3). The results showed that in both 8- and 12-week protocols, Bax gene expression significantly decreased in the CR+AE and AE groups compared to the control group (P < 0.05). No considerable difference was found between the CR, CR+AE, and AE groups in both protocols. Also, the expression of Bax in the CR12, CR+AE12, and AE12 groups was reduced in the 12-week protocol compared to the 8-week groups, which was not significant (P > 0.05) (Figure 4). Histopathological observations performed by H&E staining were significantly different (P < 0.05) in terms of the percentage of fat and the level of liver inflammation between different groups (Figure 5). In both 8- and 12-week protocols, the amount of tissue damage in the CR+AE groups showed a considerable reduction in comparison to the control group (P < 0.05). Also, the amount of tissue damage was reduced in the AE12 and CR+AE12 groups compared to the AE8 and CR+AE8 groups, which was not significant (P > 0.05). No considerable difference was found between the AE and CR+AE groups in both 8- and 12-week protocols. Figure 6 shows the cross-sectional images of rat liver tissue stained with H&E. As seen, the cytoplasm is pink, and the nuclei are purple. The results of this part of the study were seen in the sham group. The liver cells were observed with normal shape, and in the following, liver cells were observed in the form of liver plates. In the spaces between the liver plates, liver sinusoids were found from the periphery of the lobule to the lobule central vein. A hepatic triad was observed around the lobule and central vein in the center of each lobule. The percentage of liver cells without nuclei with apoptotic appearance was 5% in this group. Moreover, the investigation of the images in the 12-week high-fat control group revealed that the percent of vacuolated hepatic cells with unclear nuclei and membranes increased considerably in comparison to the other studied groups. Due to the destruction of a part of the liver tissue, the dead cells were less pigmented than living cells, and this amount of tissue discoloration was observed in this group of about 50%. Accumulations of lymphocytes and blood cells were found in the destroyed spaces caused by dead cells. The images of the 8-week high-fat control group revealed that the proportion of vacuolated hepatic cells with unclear nuclei was lower than that of the 12-week group. Also, the examination of the images related to the 12-week CR+AE group showed that the hepatic cells possessed a normal appearance. In the interlobular space, there was a central vein with a normal appearance, the hepatic triad, and between each lobule. Kupffer cells were observed around sinusoidal vessels. A small number of liver cells were observed without nuclei and with a similar appearance to apoptotic cells. The percent of cells without nuclei was observed in about 20% of the total tissue. The 8-week CR+AE group had a lower percentage of improvement than the 12-week group. In the 8- and 12-week AE and CR groups, the amount of vacuolated hepatic cells with nuclear membrane and unclear nucleus decreased compared to the control group, but the percentage of dead cells was higher compared to the 8 and 12-week CR+AE groups (Figure 6).
The mean and SD of Bcl-2 gene expression. CR, caloric restriction; AE, aerobic exercise; SD, standard deviation; ns, non significant. *: The significant difference at the level of P < 0.05. Blue lines: Intergroup comparison of the 12-week protocol; red lines: Intergroup comparison of the 8-week protocol; black lines: Comparison between the 8- and 12-week protocols.
The mean and SD of Bax gene expression. CR, caloric restriction, AE, aerobic exercise, SD, standard deviation, ns, non significant. *: The significant difference at the level of P < 0.05. Blue lines: Intergroup comparison of the 12-week protocol; red lines: Intergroup comparison of the 8-week protocol; black lines: Comparison between the 8- and 12-week protocols.
The mean and SD of liver inflammation in 8 and 12 weeks. CR, caloric restriction; AE, aerobic exercise; SD, standard deviation; ns, non significant. *: The significant difference at the level of P < 0.05. Blue lines: Intergroup comparison of the 12-week protocol; red lines: Intergroup comparison of the 8-week protocol; black lines: Comparison between the 8- and 12-week protocols.
5. Discussion
Recently, the people in the world with NAFLD account for over 1:4 of the total population (18). Currently, due to the lack of detailed information about the underlying mechanisms of this disease, no definitive drug exists to treat NAFLD (19). Thus, the treatment of NAFLD and NASH is focused on lifestyle modification similar to the recommended treatment for metabolic syndrome, including weight loss, dietary changes, and increased physical activity (1). The results of a recent meta-analysis study showed that exercise intervention with diet can be effective in improving and treating NAFLD (20). In the NAFLD progression, hepatic steatosis is the initial factor, and inflammatory response and oxidative stress are closely associated with insulin resistance as the pathological and physiological basis of the NAFLD progression. Owing to genetic susceptibility, obesity, overweight, and other causes, the body produces insulin resistance, resulting in liver steatosis, and excessive accumulation of free fatty acid in the liver causes lipotoxicity, leading to the damage of liver cells by endoplasmic reticulum stress and oxidative stress until apoptosis (18). In the present work, the NAFLD rat model was developed by feeding rats an HFD for eight weeks. They were then exposed to the AE and CR after grouping for eight and twelve weeks. In both 8- and 12-week protocols, the Bax expression in the exercised groups (AE, CR+AE) revealed a considerable decrease than the control group. In fact, the groups that had only a high-fat diet had significantly increased Bax expression than the other groups (P < 0.05). Also, no considerable difference was seen between the CR, CR+AE, and AE groups in both protocols. The comparison between the 8- and 12-week protocol indicated the expression of Bax in the CR12, CR+AE12, and AE12 groups was reduced in the 12-week protocol compared to the 8-week group, which was not significant (P > 0.05) (Figure 4). The results of Bcl-2 (Figure 3) showed that in both 8- and 12-week protocols, Bcl-2 gene expression significantly increased in the CR+AE and AE groups compared to the control group (P < 0.05). No considerable difference was found between the CR, CR+AE, and AE groups in both protocols. Also, the comparison between 8- and 12-week protocols showed that the expression of Bcl-2 increased in the CR12, CR+AE12, and AE12 groups in the 12-week protocol compared to the 8-week groups, which was not significant (P > 0.05). These findings were in line with the study of Ruan et al. who investigated the effect of exercise with different intensities on the apoptosis of liver cells in rats with fatty liver caused by a high-fat diet. It was reported that exercise with various intensities could increase the levels of Bcl-2 and also reduce Bax, apoptosis, and ultimately liver damage (21). Also, the study of Mehboodi et al. demonstrated that 6 weeks of swimming training in old rats compared to the control group could decrease the expression of Bax gene and increase Bcl-2 levels (22). The findings of Kim et al.'s study showed that 6 weeks of treadmill exercise could suppress Bax while increasing Bcl-2 expression in mice with liver injury (23). Hematoxylin-eosin findings (Figure 5) showed that inflammation significantly increased in both protocols in the control group in comparison to the sham group. Also, liver inflammation in the CR+AE group was significantly reduced in both 8- and 12-week protocols compared with the control group. The AE and CR groups did not show a significant decrease compared to the control group. Also, no significant differences were observed between AE and CR and CR+AE groups. Similarly, the findings did not show a considerable difference between the AE12, CR12, CR+AE12 and AE8, CR8, CR+AE8 groups, which was in line with the study results of Fredrickson et al. (24), van der Windt et al. (25), and Wu et al. (26).
5.1. Conclusions
Our data suggest that both exercise protocols, regardless of the duration of the exercise period, lessen hepatic inflammation and apoptosis during NASH. Also, exercise in combination with calorie restriction can be more effective in controlling inflammation and apoptosis of liver cells.