1. Context
With the emergence of the COVID-19 pandemic and the subsequent published statistics of its significant morbidity and mortality from the very first months of the onset of the disease, the efforts of researchers and medical staff were directed to reduce the severity of the disease by providing effective treatment protocols, developing written guidelines for the care of patients, and also the preparation of effective vaccines to prevent the occurrence of disease and its severity (1, 2). In less than two years after contracting this disease, effective vaccines against the disease-causing virus of various types of killed virus, weakened virus or using the virus genome were prepared and offered commercially, which resulted in their use in almost all human societies and led to limiting the mortality of patients, reducing the successive waves of the disease, and successfully controlling the complications of the disease (3-5). However, since the use of these vaccines, reports of their significant side effects have been published, both early and delayed, and some of these side effects were sometimes associated with morbidity and even mortality of patients (6). Annoying headaches, thromboembolic events, cerebrovascular events, and even ischemic heart disease and acute myocardial infarction have been potential side effects reported following the use of these types of vaccines. Even in some cases, especially cardiac ischemic events, no specific traces were found for the pathophysiological interpretation of these results (7). Sometimes, the occurrence of myocardial infarction within the first 24 hours after the injection of the vaccine in patients who had no history of cardiovascular diseases or their underlying risk factors was predictable (8). Some researchers believe that the cause of such a heart complication is the direct invasion of the virus into the myocardial tissue and its penetration through specific receptors (ACE II) located in the myocardium, resulting in the occurrence of myocarditis and cardiomyopathy (9). Some consider the coronary vessels as the direct target tissue of the virus and as a result of damage to the vascular wall, endothelial dysfunction, formation of atherosclerotic plaque, and, therefore, coronary artery stenosis and cutting (10). However, the reasons for the occurrence of such acute vascular events are still unclear, and its exact pathophysiology is still uncertain. In addition, the published reports have been limited mainly in the form of case reports of patients.
2. Objectives
The current systemic review tried to review and summarize the published evidence and documents regarding the occurrence of myocardial infarction following various types of anti-COVID-19 vaccines and, finally, take steps to clarify the causes of such incidents.
3. Materials and Methods
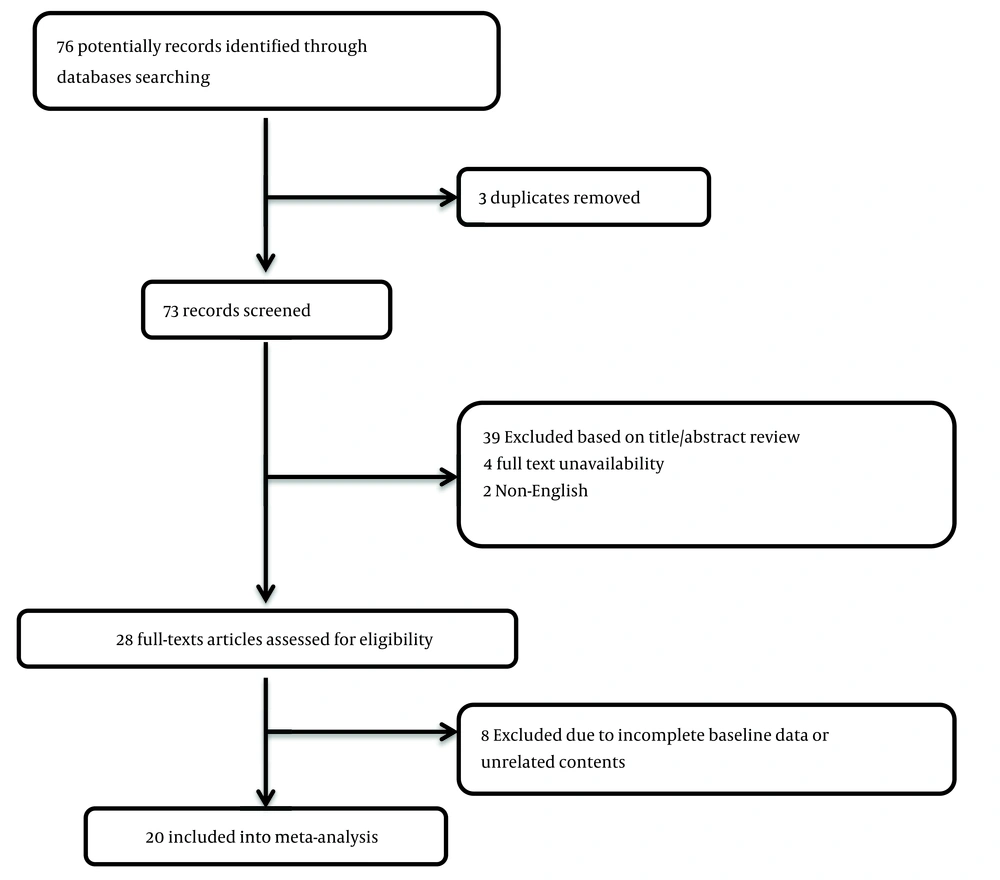
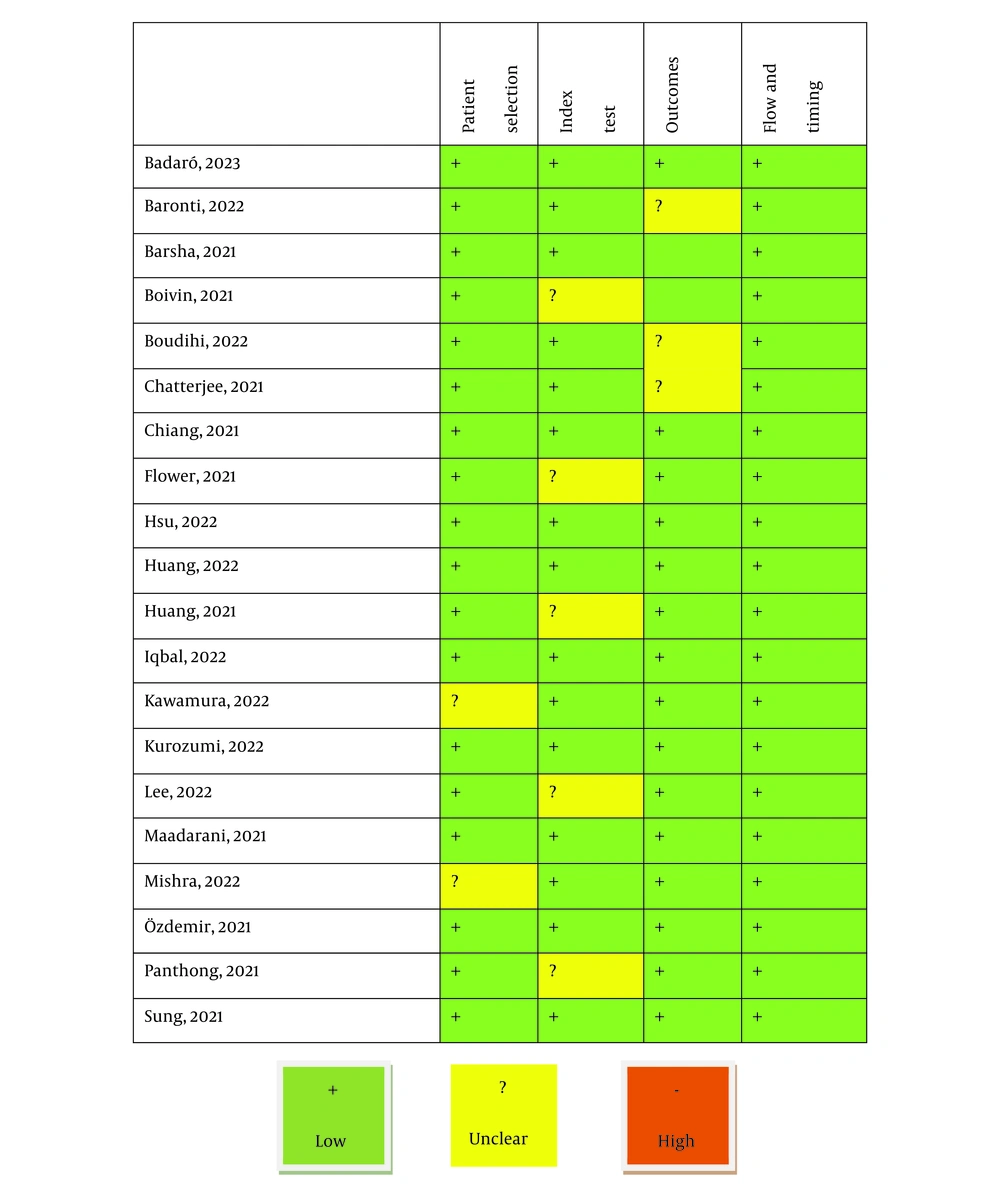
We planned the current systematic review based on the guidelines for the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) (11). First, the study questions and suggestions were developed, including "What is the prevalence of myocardial infarction following the use of COVID-19 vaccination?" "Which types of vaccines have been associated with a higher risk of this complication?" "What was the time interval between the vaccination and the occurrence of this event?" and "What could be the possible mechanism of this cardiovascular accident?" In the next step, the relevant article databases, including Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane, were comprehensively searched by the two blinded researchers for all eligible studies based on the considered keywords of “COVID-19”, “vaccine”, “prevalence”, “myocardial infarction”, “coronary artery”, “atherosclerosis”, “virus”, and “myocarditis”. Resolving disagreements was achieved by reaching a consensus between the parties involved or by seeking the assistance of a neutral third party. To retrieve the studies, the inclusion criteria were taken into consideration.: (1) The studies finally assessed were those described the cases who had no previous history of cardiovascular disease or underlying disorder risk profiles that suffered myocardial infarction following the inoculation of the COVID-19 vaccine; (2) the studies were restricted to the English language unless it was possible to translate the original version of the article completely and fluently; (3) the studies with unclear or irreproducible results were all excluded; (4) full manuscript access was needed, unless abstract data are sufficient for analysis or the full text of the article was provided by sending an email and requesting to release it; (5) any related review articles were also excluded from the study. As shown in the study selection flow diagram (Figure 1), at first, the database search yielded 76 articles. After identifying three duplicated articles, 73 records were primarily under-screened. Through the evaluation of the titles and abstracts, 45 records were excluded, leaving 28 citations for further assessment. However, 8 of these citations were also eliminated due to incomplete data and contents. Ultimately, 20 articles (February 2020 to October 2023) were deemed eligible for the final analysis (10, 12-30) (Table 1). The final assessment of study quality, the applicability of primary diagnostic accuracy studies, and the risk of bias were assessed based on the QUADAS-2 tool. It assesses study quality and bias risk by looking at patient selection, index test, reference standard, and flow/timing. Four phases have been defined in the application of QUADAS-2, including (1) summarizing the questions reviewed; (2) tailoring the tool to the review and producing review-specific guidance; (3) constructing a flow diagram for the primary study; and (4) assessing the risk of bias and concerns regarding applicability. All 16 studies had a low risk of bias and were of good quality, making the pooled results persuasive (Figure 2). To reach the final judgment on the results, the comprehensive meta-analysis (CMA) software version 3.0 (Biostat, Englewood, NJ 07631 USA) was employed.
| Authors | Year | Country | Study Type | Type of Vaccine | Vaccine Time | Time of Myocardial Infarction Occurrence After Vaccination | Type of Myocardial Infarction | Gender | Age | Medical History | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Badaro et al. (10) | 2023 | Brazil | Case report | Pfizer | First | 8 hours | STEMI | Male | 23 | WPW | Survived |
| Baronti et al. (12) | 2022 | Italy | Case series | Pfizer | First | 48 hours | STEMI | Male | 69 | DM, COPD | Died |
| 8 hours | STEMI | Male | 58 | - | Died | ||||||
| 21 days | STEMI | Male | 76 | - | Died | ||||||
| 72 hours | STEMI | Male | 68 | HTN | Died | ||||||
| 24 hours | STEMI | Female | 50 | Smoking | Died | ||||||
| Barsha et al. (13) | 2021 | Bangladesh | Case report | Moderna | First | 48 hours | NSTEMI | Male | 77 | DM, COPD | Survived |
| Boivin and Martin (14) | 2021 | USA | Case report | Moderna | First | 1 hour | STEMI | Female | 96 | HTN | Survived |
| Boudihi et al. (15) | 2023 | Morocco | Case report | Sinopharm | Second | 24 hours | STEMI | Male | 23 | - | Survived |
| Chatterjee et al. (16) | 2021 | India | Case report | Covishield | First | 48 hours | STEMI | Male | 63 | - | Survived |
| Chiang et al. (17) | 2021 | Taiwan | Case report | AstraZeneca | First | 8 days | STEMI | Female | 75 | RF | Survived |
| Flower et al. (18) | 2021 | UK | Case report | AstraZeneca | First | 8 days | STEMI | Male | 40 | - | Survived |
| Hsu et al. (19) | 2022 | Taiwan | Case report | AstraZeneca | First | 9 days | STEMI | Male | 33 | Obesity, HLP | Died |
| Huang et al. (20) | 2022 | Taiwan | Case report | AstraZeneca | First | 72 hours | STEMI | Female | 78 | DM, RF | Survived |
| AstraZeneca | First | 72 hours | NSTEMI | Male | 89 | DM, HTN | Survived | ||||
| AstraZeneca | First | 5 days | STEMI | Female | 87 | Colon cancer | Survived | ||||
| Huang et al. (21) | 2022 | Taiwan | Case report | AstraZeneca | First | 2 hours | STEMI | Male | 60 | - | Survived |
| Iqbal et al. (22) | 2022 | Pakistan | Case report | Moderna | First | 3 hours | NSTEMI | Male | 61 | DM, HTN, IHD | Survived |
| Kawamura et al. (23) | 2023 | Japan | Case report | Pfizer | Second | 19 hours | STEMI | Female | 76 | HTN, HLP, asthma | Survived |
| Kurozumi et al. (24) | 2022 | Japan | Case report | Pfizer | Second | 15 min | STEMI | Female | 70 | DM, HTN, HLP | Survived |
| Lee et al. (25) | 2022 | Taiwan | Case report | AstraZeneca | First | 7 days | STEMI | Male | 85 | RF | Survived |
| Maadarani et al. (26) | 2021 | Kuwait | Case report | AstraZeneca | First | 1.5 hours | STEMI | Female | 62 | DM, HTN, HLP | Survived |
| Mishra et al. (27) | 2022 | India | Case report | Covishield | First | 12 hours | STEMI | Male | 68 | HTN | Survived |
| Ozdemir et al. (28) | 2021 | Turkey | Case report | Sinovac | First | 15 min | STEMI | Female | 41 | - | Survived |
| Panthong et al. (29) | 2022 | Thailand | Case report | CoronaVac | Second | 18 hours | STEMI | Male | 48 | DM, HTN, HLP | Survived |
| CoronaVac | First | 12 hours | NSTEMI | Male | 50 | IHD | Survived | ||||
| Sung et al. (31) | 2021 | USA | Case report | Pfizer | First | 24 hours | STEMI | Female | 68 | HTN, HLP, smoking | Survived |
| Pfizer | First | 24 hours | NSTEMI | Male | 42 | HLP | Survived |
The Details of the Studies Evaluated
4. Results
To evaluate the frequency of MIS-C-related cardiac abnormalities using selected keywords, 20 studies were analyzed (including 28 patients, 18 men, and 9 women consisting of individuals aged between 23 and 96 years, with an average age of 62 years). Publications from February 2020 to October 2023 were evaluated, with contributions from multiple countries (Table 1). Among the 20 published studies, 19 were presented as case reports, and one was presented as a case series. Reports of myocardial infarction following vaccination almost included both Eastern and Western countries, and it was independent of the type of society. However, the occurrence of acute myocardial infarction was mostly related to SARS-CoV-2-based messenger RNA (mRNA) and viral vector vaccines. In this regard, of 28 vaccinated patients who suffered post-vaccination myocardial infarction, this cardiac event occurred following vaccination by AstraZeneca vaccine in 9 patients, and 10 events occurred following vaccination by Pfizer-BioNTech COVID-19 Vaccine, and 3 cases by Moderna, 2 cases by coronaVac, 2 cases by Covishield, 1 case by Sinovac, and 1 case by Sionopham. Regarding the time of occurrence of myocardial infarction, this cardiac attack occurred after the first vaccination in 24 out of 28 patients affected and after the second time in other patients (myocardial infarction after AstraZeneca was inclusively after the first dose, but myocardial infarction following other vaccines was after the first or second dose. The time of occurrence of myocardial infarction was also very different between different types of vaccines and varied between 15 minutes and 21 days after vaccination (average 2.8 days after vaccination). Most of the myocardial infarctions that occurred after vaccination were of the ST-segment elevation type (STEMI), which indicated the extent and severity of myocardial involvement following the inoculation of vaccines. More than two-thirds of myocardial infarction cases occurred in patients who had significant clinical history, including hypertension (32%), diabetes mellitus (28%), hyperlipidemia (25 %), history of renal failure (11%), history of coronary heart disease (7%), and history of smoking (7%). Concerning survival outcomes following post-vaccination myocardial infarction, 22 out of 28 patients survived and were discharged from the hospital in good condition; however, 6 cases died within hospitalization.
5. Discussion
Twenty studies were analyzed with 28 patients who experienced myocardial infarction after receiving COVID-19 vaccines. Reports occurred in various countries, with most cases following mRNA and viral vector vaccines. Myocardial infarction occurs after the first vaccination in most cases, with a time frame of 15 minutes to 21 days after vaccination. Most affected patients had significant clinical history, but 22 survived, and 6 died during hospitalization. Most of the deaths due to COVID-19 are reported due to extensive pulmonary involvement and decreased arterial oxygen saturation, followed by cardiac complications, especially ischemic heart attacks and cardiomyopathy (32-34). It is interesting to note that these deaths occurred not only after contracting COVID-19 but, in some cases, after vaccination against the disease, and most of these deaths occurred shortly after the vaccination and in the form of acute ischemic heart attacks. Information about the causes of myocardial infarction after vaccination against COVID-19 is limited and remains at the level of hypothesis. Vaccination can cause a prothrombotic condition similar to autoimmune heparin-induced thrombocytopenia (35). In other words, stress caused by the vaccination against COVID-19 can result in demand ischemia, resulting in cardiac events (36). Myocardial infarction after vaccination can be caused by Kounis syndrome, which is an allergic reaction that triggers vasospasm (37). According to our finding that most patients had a positive clinical history in terms of cardiovascular risk factors, the existence of the same underlying risk factors can be considered as a risk profile for the occurrence of acute myocardial infarction after vaccination. The existence of the same underlying risk factors can be considered as a risk profile for the occurrence of acute myocardial infarction after vaccination. But whether the viral vector or RNA virus used in the vaccine can be a trigger for inflammatory reactions and damage to the myocardium is still a hypothesis. This hypothesis becomes stronger when most of the cardiovascular attacks occurred after vaccination related to vaccines based on virus vectors or virus genomes. Moreover, vaccine-related thromboembolic events have been observed with such vaccines (38, 39), and it is strongly hypothesized that the thrombotic reaction following vaccination, especially following vaccines based on the virus genome, can be a trigger for ischemic heart attacks. As recently described, thrombosis and thrombocytopenia occurred 6 to 24 days after the first dose of the COVID-19 vaccination (40). In general, although the occurrence of acute myocardial infarction has been an uncommon phenomenon following vaccination against COVID-19 (31) (prevalence less than three-thousandths of a percent), to achieve high safety of these types of vaccines, biotechnological modification of the relevant vaccines is still necessary.
5.1. Suggestions
In the future, the relationship between the 1st, second, and third doses of vaccines and myocardial infarction could be evaluated in a wide range and much more research.