1. Background
Levofloxacin is a broad-spectrum fluoroquinolone antibiotic with activity against both Gram-positive and Gram-negative bacteria, as well as atypical pathogens (1). It finds extensive use in the treatment of various infections, including respiratory tract infections, urinary tract infections (UTIs), skin and soft tissue infections, and exposure to anthrax (2). However, levofloxacin also carries potential adverse effects, such as tendon rupture, nerve damage, cardiac arrhythmias, and Clostridioides difficile infection (3). Reports from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) indicate that neuropsychiatric toxicity, long-term disability, and aortic dissection/aneurysms can occur with all fluoroquinolones. These disabilities and neuropsychiatric symptoms may manifest after a single dose or several months following fluoroquinolone use (4). Fluoroquinolones have been prescribed in Iran for approximately 50 years, and recent studies have focused on the sensitivity and resistance patterns of Escherichia coli to levofloxacin and other fluoroquinolones (5).
The world is currently grappling with the threat of antibiotic-resistant bacteria, which can rapidly evolve into superbugs (3). Antibiotic resistance stands as one of the three most significant public health challenges of this century (6). Strategies that encompass substantial reductions, changes in antibiotic use, and stewardship programs are imperative to reverse the trends of drug resistance. Appropriate antibiotic prescriptions play a pivotal role in controlling antibiotic resistance (7). The World Health Organization (WHO) emphasizes that data on anti-microbial usage can provide insights into how exposure to anti-microbials influences the emergence of anti-microbial resistance (8).
The highest reported resistance rate to levofloxacin has been among patients with UTIs caused by E. coli isolates in the western regions of Iran. There is limited information available about the anti-microbial resistance of E. coli to levofloxacin in different geographical areas of Iran (9-11). A review study revealed that resistance of E. coli to fluoroquinolones has increased in recent times, substantially limiting the use of this class of drugs. These findings, coupled with emerging data on inappropriate administration and toxicity, have restricted its clinical utility (12).
2. Objectives
Given the lack of comprehensive information on the consumption of fluoroquinolones and their role in treating bacterial infections in Iran, our study aimed to evaluate the prescription patterns of levofloxacin based on international standards.
3. Methods
3.1. Study Design
This retrospective cross-sectional descriptive study aimed to investigate the prescription patterns of levofloxacin at Imam Khomeini Hospital in Ahvaz, Iran, during the first 6 months of 2020. The study was conducted using a census method, including all patients who received levofloxacin during the specified time period.
In our hospital, a rational prescription form must be completed and recorded in the pharmacy's health information system (HIS) every time levofloxacin is prescribed. Therefore, we relied on HIS for data collection. Patients who received less than 2 consecutive doses of levofloxacin due to reasons such as discontinuation of antibiotics, discharge, death, or changes in their treatment regimen were excluded from the study.
3.2. Data Collection Form
The data collection questionnaire included patient demographic information (age, sex, admission and discharge dates, or date of death), diagnosis, laboratory test results, details regarding levofloxacin administration, and information about the patient's condition. All of this information was extracted from the patients' medical records.
During the evaluation process, we considered factors such as the correct dosage, frequency of administration, drug interactions, and adverse effects associated with levofloxacin. We utilized the standard treatment guidelines developed by the FDA to determine whether the use of the drug was rational or irrational (13). The form of drug administration (intravenous or oral), as well as the dosage and duration of use, were evaluated based on guidelines for specific diseases (including wound infections, respiratory infections, Helicobacter pylori treatment, UTIs, etc.).
3.3. Statistical Analysis
Descriptive statistics, including frequencies and percentages, were employed to analyze the collected data concerning demographic and clinical variables. Data analysis was performed using SPSS v. 22 (IBM Corporation, Armonk, NY).
4. Results
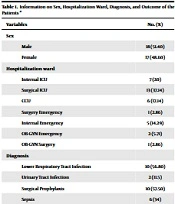
A total of 35 patients received levofloxacin over a 6-month period. The average age of these patients was 55.17 ± 20.36 years, with the youngest being 13 and the oldest 90 years old. Among them, 17 patients (48.6%) were women, and 18 (51.4%) were men. The majority of patients were admitted to the surgical intensive care unit (ICU) and coronary care unit (CCU). The most common medical diagnoses among the patients were lower respiratory tract infection and surgical prophylaxis. Furthermore, 68.57% of the patients were discharged, while 25.71% of them passed away during hospitalization (Table 1).
| Variables | No. (%) |
|---|---|
| Sex | |
| Male | 18 (51.40) |
| Female | 17 (48.60) |
| Hospitalization ward | |
| Internal ICU | 7 (20) |
| Surgical ICU | 13 (37.14) |
| CCU | 6 (17.14) |
| Surgery emergency | 1 (2.86) |
| Internal emergency | 5 (14.29) |
| OB-GYN emergency | 2 (5.71) |
| OB-GYN surgery | 1 (2.86) |
| Diagnosis | |
| Lower respiratory tract infection | 10 (56.80) |
| Urinary tract infection | 2 (11.5) |
| Surgical prophylaxis | 10 (57.50) |
| Sepsis | 6 (34) |
| Other | 7 (50.20) |
| Outcome | |
| Discharge | 24 (68.57) |
| Death | 9 (25.71) |
| Quitting against medical advice | 2 (5.71) |
Abbreviations: ICU, intensive care unit; CCU, coronary care unit; OB-GYN, obstetrics and gynecology.
a Other diagnosis: Abscess, diabetic foot infection, pseudomembranous colitis, hepatitis
The average prescribed dose of levofloxacin was 3.20 ± 2.859 grams per patient, with the minimum dose being 500 milligrams and the maximum dose reaching 11.50 grams (Table 2). The average duration of treatment was 4.26 ± 3.81 days, ranging from a minimum of 1 day to a maximum of 16 days.
| Diagnosis | Dosage (g), Mean ± SD | Treatment Period (d), Mean ± SD |
|---|---|---|
| Lower respiratory tract infection | 3.05 ± 3.004 | 4.07 ± 4.006 |
| Urinary tract infection | 7.07 ± 6.5 | 9.42 ± 8.67 |
| Surgical prophylaxis | 3.05 ± 1.81 | 4.07 ± 2.42 |
| Sepsis | 1.58 ± 0.665 | 2.11 ± 0.886 |
| Other | 4.07 ± 3.45 | 5.43 ± 4.61 |
a Other diagnosis: Abscess, diabetic foot infection, pseudomembranous colitis, hepatitis.
Based on the guidelines, the use of levofloxacin was considered rational in 21 cases (60%) and irrational in 14 cases (40%). Among these 21 patients, the right dose and the correct duration of treatment were administered. In contrast, 11 patients received the correct dose but did not complete the recommended treatment duration, while 3 patients received neither the appropriate dose nor the recommended duration of treatment. Levofloxacin resistance was observed in 6 patients (17.1%) in this study, with a mortality rate of 50% among the resistant group compared to 20.7% in the non-resistant group. Among the 6 resistant patients, 2 had Acinetobacter, 3 had E. coli, and 1 had Klebsiella resistant to levofloxacin in their smears.
5. Discussion
To the best of our knowledge, antibiotic resistance stands as 1 of the 3 most critical public health challenges (6). Implementing strategies that involve significant reductions, changes in antibiotic utilization, and stewardship programs is crucial to counteract the trends of drug resistance. Appropriate antibiotic prescriptions play a vital role in controlling antibiotic resistance (7). In this study, we evaluated the prescription pattern of levofloxacin, a commonly used antibiotic in patients, based on international standards. Our results revealed that the average prescribed dose of levofloxacin was 3.20 ± 2.85 grams per patient. Notably, Rungkitwattanakul et al. found that the effective dose of levofloxacin (1,750 mg on day 1, followed by 1,500 mg every 24 hours) significantly exceeded the maximum FDA-approved doses (14). Similarly, in a study by Lewis et al., successful levofloxacin doses (2,000 mg loading dose, 1 000 mg every 24 hours) were well above the FDA-approved maximum doses (15).
We also found that the average duration of levofloxacin treatment was 4.26 ± 3.813 days, ranging from a minimum of 1 day to a maximum of 16 days. In comparison, Garcia-Vidal et al. reported a shorter ICU length of stay by 4.4 days in the levofloxacin group in their study comparing levofloxacin with other antibiotics (16). Additionally, Yadegarynia et al. noted that levofloxacin demonstrated better performance in terms of hospitalization duration compared to ceftriaxone and azithromycin (17). However, another study observed a longer treatment duration for levofloxacin, around 7 - 10 days (18), which exceeded the duration in our study, possibly due to differences in the primary diagnoses of patients.
Based on the guidelines, our results indicated that the use of levofloxacin was considered rational in 21 cases (60%) and irrational in 14 cases (40%). In contrast, Werida et al., who evaluated levofloxacin utilization in an ICU, found that the majority of patients (78.4%) received levofloxacin as empirical treatment, with 27.9% of patients receiving inappropriate levofloxacin treatment (18).
In our study, resistance to levofloxacin was observed in 6 patients (17.1%), with a 50% mortality rate within this group. Caliskan et al. found that 29.5% of the patients examined had levofloxacin resistance (19). Karczewska et al. reported levofloxacin resistance rates of 12% and 38% in treatment-naïve and previously levofloxacin-treated patients, respectively (20). Furthermore, a study noted high levofloxacin and ciprofloxacin resistance rates in Iran (72.5%) and India (50%), whereas Western and Southern Asia exhibited lower levofloxacin resistance rates (21). These discrepancies in statistics may be attributed to differences in sample size and investigation duration. Among the 6 resistant patients, 2 of them had Acinetobacter, 3 had E. coli, and 1 had Klebsiella resistant to levofloxacin in their smears. A study found a significant correlation between levofloxacin use and the incidence of nosocomial fluoroquinolone-resistant E. coli isolates, suggesting that limiting levofloxacin consumption could reduce the incidence of fluoroquinolone-resistant E. coli (22). The results of a review study indicated a current increase in E. coli resistance to fluoroquinolones, substantially restricting the use of this drug class. These findings, combined with emerging data on inappropriate administration and toxicity, have limited its clinical utility (12). Zhang et al. recommended combining levofloxacin with a bactericidal antibiotic like ampicillin and sulbactam to combat Acinetobacter resistance (23).
It is worth noting that this study has some limitations, including its retrospective nature, limited time frame, and small sample size. Conducting this study over an extended period with a larger sample size may yield more reliable results regarding the status of levofloxacin administration in patients and the appropriate strategies to follow.
5.1. Conclusions
According to our findings, levofloxacin was prescribed correctly in over 80% of cases, and the prescribed dose of the drug was used correctly in more than 90% of cases. However, the duration of treatment was not administered correctly in nearly half of the cases. Apart from contributing to drug resistance, these issues may lead to increased antibiotic resistance over time and pose significant challenges for patients.