1. Background
The coronavirus disease 2019 (COVID-19) remains a global concern and continues to claim victims. In severe cases, as with other serious illnesses, there is evidence of changes in serum complement levels (1). Respiratory deterioration in this disease has been associated with increased viral loads and an inadequate immune response (2). The complement system plays a crucial role in preventing various infectious strains by activating both the innate and acquired immune systems (3). It comprises several proteins, and the activity of each pathway ultimately leads to the cleavage and conversion of complement components, such as complement C3 (4). There is controversy surrounding the role of the complement system in COVID-19. Some studies have shown an increase in complement levels (5); however, others have demonstrated a decrease (6, 7), and some reports have indicated no changes (8).
The activation of C3 exacerbates disease severity in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated acute respiratory distress syndrome (ARDS). C3-deficient mice infected with SARS-CoV-2 exhibited almost normal respiratory function. These mice had fewer neutrophils and inflammatory monocytes infiltrating the lung tissue, resulting in lower levels of cytokines and chemokines in the lungs and serum (9).
Complement activity increases in hospitalized COVID-19 patients and is significantly associated with markers of inflammation. Patients with lower C3 levels and high complement activity had a higher mortality rate than those with normal complement activity and C3 levels (7).
Saliva has been used for years to assess human health and diseases (10-17), offering numerous advantages as a non-invasive, painless, safe, and easily accessible sample. Its sampling can be repeated comfortably.
2. Objectives
This study aimed to evaluate the levels of C3 and C4 in both saliva and serum of patients with severe and critical COVID-19 and healthy individuals. The goal was to determine whether COVID-19 infection affects the levels of C3 and C4 in both saliva and serum.
3. Methods
A cross-sectional study was conducted at Shahid Mohammadi Hospital in Bandar Abbas, Iran. The study was approved by the Ethics Committee of AJA University of Medical Sciences, Tehran, Iran (IR.AJAUMS.REC.1401.116). This prospective monocentric study included a total of 58 patients. There were 22 patients in the critical stage (mean age: 40.2 ± 10.9 years) and 36 patients in the severe stage (mean age: 44.3 ± 13.3 years) who were admitted to the COVID-19 unit.
The control group consisted of 22 individuals (mean age: 40.1 ± 5.5 years), including 9 males and 13 females, who were selected from healthcare workers undergoing annual occupational health assessments. Participants who were less than 18 years old and had autoimmune diseases, such as systemic lupus erythematosus, autoimmune liver disease, and Sjogren’s syndrome, were excluded from the study. All relevant background information was evaluated and recorded at the time of admission (Table 1). The mean ± standard deviation (SD) ages were 40.1 ± 5.5 years for healthy individuals, 40.2 ± 10.9 years for those with severe COVID-19 infection, and 44.3 ± 13.3 years for those in critical condition.
| Variables | Severe COVID-19 | Critical COVID-19 | P-Value |
|---|---|---|---|
| Gender (female/male) | 14/22 | 13/9 | 0.134 |
| Red blood cell (per μL) | 4935500 ± 85180 | 4973600 ± 157950 | 0.817 |
| White blood cell (per μL) | 5050 ± 347 | 7360 ± 909 a | 0.005 |
| Neutrophil (%) | 69.7 ± 1.9 | 74.4 ± 3.2 | 0.195 |
| Lymphocyte (%) | 25.0 ± 1.7 | 21.0 ± 2.6 | 0.202 |
| Neutrophil/lymphocyte ratio | 3.94 ± 0.47 | 5.32 ± 0.84 | 0.128 |
Demographic Characteristics of Study Patients
Serum and whole saliva samples were collected within 24 - 48 hours of admission. Unstimulated saliva was collected from participants between 9 and 11 a.m. The participants were advised not to eat, drink, or smoke for at least 2 hours before sample collection. The patients were instructed to sit comfortably and spit saliva into a plastic falcon tube using a funnel. On the morning of saliva collection, 2 mL of venous blood was collected from all participants and placed in gel clot tubes. The samples were then centrifuged at 3000 rpm for 10 minutes. The supernatants from both saliva and serum samples were stored at -70°C. After the completion of sample collection, all samples were thawed and sent to the laboratory for the determination of serum and salivary levels of C3 and C4 proteins using the immunoturbidimetric method (Biorexfars, Shiraz, Iran), following the manufacturer’s instructions.
3.1. Statistical Analysis
The data are presented as mean ± standard error of the mean (SEM) and were analyzed using one-way analysis of variance (ANOVA), followed by the Student-Newman-Keuls test as a post hoc analysis.
4. Results
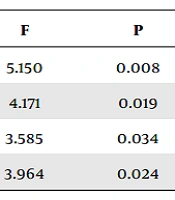
Significant differences in the mean levels of saliva C3 and C4 were observed among the groups (Table 2). Saliva C3 and C4 levels were lower in patients with severe and critical COVID-19 than in healthy individuals. However, there was no significant difference between patients with severe and critical COVID-19. Similarly, significant differences in the mean levels of serum C3 and C4 were observed among the groups (Table 2). Serum C3 levels were lower in critical COVID-19 patients than in healthy individuals, with no significant difference between severe COVID-19 patients and healthy individuals. Serum C4 levels were lower in critical COVID-19 patients than in both healthy individuals and severe COVID-19-infected groups; nevertheless, there was no significant difference between severe COVID-19 patients and healthy individuals.
| Variables | Control | Severe COVID-19 | Critical COVID-19 | F | P |
|---|---|---|---|---|---|
| Saliva C3 (mg/dL) | 0.22 ± 0.06 | 0.08 ± 0.02 b | 0.08 ± 0.02 b | 5.150 | 0.008 |
| Saliva C4 (mg/dL) | 0.07 ± 0.04 | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 4.171 | 0.019 |
| Serum C3 (mg/dL) | 132.3 ± 5.3 | 120.6 ± 4.3 | 104.3 ± 10.1 b | 3.585 | 0.034 |
| Serum C4 (mg/dL) | 31.9 ± 1.7 | 29.2 ± 1.9 | 21.8 ± 2.8 b, c | 3.964 | 0.024 |
Serum and Saliva Levels of C3 and C4 in Coronavirus Disease 2019 Infection a
5. Discussion
Severe acute respiratory syndrome coronavirus 2 might infect the salivary glands, potentially affecting saliva composition. In this study, the levels of C3 and C4 in the serum and saliva of healthy individuals and patients with severe and critical COVID-19 were evaluated. The results demonstrated that the levels of C3 and C4 in patients' saliva were significantly lower than those in the healthy participants. Furthermore, the serum levels of C3 and C4 were lower in individuals with critical COVID-19 infection than in healthy individuals. Additionally, serum C4 levels were lower in individuals with critical COVID-19 infection than in those with severe COVID-19 infection. However, there was no significant difference in serum C3 and C4 levels between individuals with severe COVID-19 infection and healthy individuals.
The innate immune system plays a pivotal role in responding to viral infections. Previous studies have shown that the complement system significantly contributes to immune activation in patients with human immunodeficiency virus (HIV). In sepsis, the virus primarily activates the complement system through the classical pathway, leading to increased C4 consumption (18). Similarly, complement activation occurs during COVID-19 infection (19). Several reports have indicated a reduction in serum C3 and C4 levels in SARS-CoV-2-infected patients (1, 20-22). It has also been reported that serum C3 and C4 levels are reduced in other viral diseases, such as hepatitis B (23). It has been reported that C3 significantly correlates with inflammatory markers, such as white blood cell count, C-reactive protein, ferritin, D-dimer, and albumin. Therefore, with higher COVID-19 severity, there is a decrease in the concentrations of C3 and C4, likely due to complement activation and an increase in mortality (24). These results align with the results of the present study, although there is an opposing report suggesting higher levels of complement components C3 and C4 than normal ranges in COVID-19 patients (5).
The current study revealed reduced salivary levels of C3 and C4 in both severe and critical SARS-CoV-2 infections. To the best of our knowledge, there have been no previous reports on C3 and C4 levels in the saliva of SARS-CoV-2-infected patients. The amount of C3 and C4 in the serum of the severe SARS-CoV-2 infections group did not decrease significantly; however, a significant decrease was observed in the saliva of these patients. Importantly, the reduction of these complements in the saliva of these patients appears to occur earlier than in the serum, suggesting that the virus might initially affect the upper respiratory system and salivary glands before impacting the rest of the body. The aforementioned findings underscore the potential utility of saliva as a diagnostic fluid for COVID-19, compared to serum (25).
The observed reduction in serum and salivary C3 and C4 levels in COVID-19 patients can be explained by two possible mechanisms. Firstly, the liver is responsible for producing these complements, and COVID-19-induced liver damage might lead to reduced C3 and C4 production. Secondly, COVID-19 infection might generate various antigen-antibody complexes that activate the complement system, resulting in the excessive consumption of C3 and C4 and eventually leading to their reduction. This finding is supported by the evidence indicating increased C3 consumption in SARS-CoV-2 infection (7).
5.1. Conclusions
It appears that both serum and salivary C3 and C4 levels decrease in patients with COVID-19 infection.