1. Background
Gastric cancer is the fourth leading cause of cancer-related mortality globally (1). Despite declining trends in recent decades, gastric cancer has become a significant concern due to its high incidence of more than 1 million cases annually, unfavorable survival rates, and considerable adverse effects on patients’ quality of life (1, 2).
According to global evidence, gastric cancer usually affects patients in their sixth to ninth decades of life and has a male predominance of affection (3). Known risk factors include obesity and gastroesophageal reflux disease in cancers of the cardia, Helicobacter pylori infection, a high salt/salt-rich diet in non-cardia cancers, and older age, male sex, and smoking for both types of gastric cancer (4, 5). Most cases are diagnosed in the advanced stages of the disease due to the long asymptomatic phase before the diagnosis (6). Surgical resection and chemoradiation, either as neoadjuvant or adjuvant therapy, are the available treatment strategies (7). Current evidence on gastric cancer patients in Iran is limited and does not provide a detailed view of the patients’ clinicopathologic characteristics and survival rates (8). The risk of gastric cancer is generally high in the northern and northwestern provinces of Iran. Therefore, it is imperative to have different health policies in place for each province, depending on their gastric cancer risk and trend. It is necessary to improve screening and education programs and conduct further epidemiological studies to reduce the incidence of gastric cancer in high-risk provinces (9). Several factors (such as genetics, lifestyle choices, medical history, smoking, alcohol consumption, obesity, chronic anemia, and type A blood groups) can increase the risk of developing gastric cancer (10).
It is crucial to have a comprehensive understanding of various factors, such as demographic characteristics of patients, clinical symptoms, laboratory data, endoscopic ultrasonography (EUS) staging, computed tomography (CT) scan findings, histopathologic subgroups, and associated risk factors to plan for reducing the incidence of gastric cancer. Therefore, this study aimed to investigate the above-mentioned factors of gastric cancer. To the best of our knowledge, such a comprehensive study has not been conducted in Iran yet.
2. Objectives
This study presents a comprehensive report on the epidemiology, pathology, imaging, laparoscopic evaluation, and surgical findings of patients with gastric cancer who were referred to a tertiary hospital in Tehran, Iran.
3. Methods
This single-center cross-sectional study was designed to investigate the epidemiologic features, pathologic grading, imaging results, and surgical findings of patients with gastric cancer who underwent laparoscopic investigation and staging of the gastric tumor in Shariati Hospital, Tehran, Iran, for 4 years (March 2018- October 2023). All gastric cancer patients who were referred to the hospital and underwent diagnostic laparoscopic staging during the study period were included. The exclusion criteria were the absence of follow-up, history of bariatric or any other major gastrointestinal surgery, prior gastrointestinal tumor, and synchronous or metachronous malignancy. The patient’s demographic data, including age, sex, weight, height, body mass index (BMI) at the beginning of the study, comorbidities, drug history, chemoradiation history, clinical presentation (signs and symptoms, including abdominal pain, weight loss, nausea, vomiting, significant weight loss, anorexia, malaise, melena, hematemesis, dysphagia, epigastric tenderness), duration of symptoms, physical examination findings, and paraclinical assessments, including laboratory, endoscopic, EUS, CT scan, diagnostic laparoscopic, and pathologic findings, were collected in a questionnaire. The patients were followed for 2 years. The treatment strategy, surgical outcomes, the patient’s death, and the cause were recorded.
3.1. Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, v. 25.0, IBM Corp Armonk, NY). The quantitative and qualitative variables were expressed as mean ± standard deviation (SD) and percentage, respectively. The Kaplan-Meier estimate was used to assess the 2-year survival. The level of significance was considered as P< 0.05.
4. Results
A total of 30 gastric cancer patients were enrolled in the study. The mean age of the sample was 62 ± 12.6 years; 76.7% of the patients were men, and 23.3% were women. The general characteristics of the participants are presented in Table 1. The most prevalent comorbidity was hypertension, followed by anemia and diabetes. Proton pump inhibitors (PPIs) were found to be the most commonly used medication chronically before surgery. There was no family history of gastric cancer among participants. Four patients were active smokers, 1 had opium addiction, and 1 had a history of chronic alcohol use. Only 1 patient (3.3%) had a neoadjuvant chemotherapy history, and none had received radiation before the surgery.
| Variables | Male | Female |
|---|---|---|
| Sex | 23 | 7 |
| Age (y) | 62.0 ± 12.6 | |
| Weight | 68.6 ± 12.45 | |
| Height | 169.9 ± 8.6 | |
| BMI | 23.74 ± 3.8 | |
| Comorbidity | Yes (%) | No (%) |
| HTN | 8 (26.7) | 22 (73.3) |
| Anemia | 7 (23.3) | 23 (76.7) |
| DM | 5 (16.7) | 25 (83.3) |
| IHD | 4 (13.3) | 26 (86.7) |
| DLD | 3 (10.0) | 27 (90.0) |
| BPH | 3 (10.0) | 27 (90.0) |
| DVT | 2 (6.7) | 28 (93.3) |
| Hepatitis B | 1 (3.3) | 29 (96.7) |
| Hyperthyroidism | 1 (3.3) | 29 (96.7) |
| PUD | 1 (3.3) | 29 (96.7) |
| Asthma | 1 (3.3) | 29 (96.7) |
| Renal Failure | 1 (3.3) | 29 (96.7) |
| Adrenal mass | 1 (3.3) | 29 (96.7) |
| CVA | 1 (3.3) | 29 (96.7) |
| Hypothyroidism | 0 | 30 (100) |
| Ascites | 0 | 30 (100) |
| Risk factors | Yes (%) | No (%) |
| Smoking | 4 (13.3) | 26 (86.7) |
| Alcohol | 1 (3.3) | 29 (96.7) |
| Opium | 1 (3.3) | 29 (96.7) |
| Family history | 0 | 30 (100) |
| Drug history | Yes (%) | No (%) |
| PPI | 7 (23.3) | 23 (76.7) |
| Anti-HTN | 5 (16.7) | 25 (83.3) |
| ASA | 4 (13.3) | 26 (86.7) |
| Anti-DM | 3 (10.0) | 27 (90.0) |
| Cardiac | 3 (10.0) | 27 (90.0) |
| Famotidine | 2 (6.7) | 28 (93.3) |
| LLD | 2 (6.7) | 28 (93.3) |
| Warfarin | 1 (3.3) | 29 (96.7) |
| Clopidogrel | 1 (3.3) | 29 (96.7) |
| Tamsulosin | 1 (3.3) | 29 (96.7) |
| Haloperidol | 1 (3.3) | 29 (96.7) |
a Data are presented as mean ± SD or count (%).
Abbreviations: Anti-DM, medication used to control diabetes; Anti-HTN, antihypertensive medication; ASA, aspirin; BMI, body mass index; BPH, benign prostate hyperplasia, cardiac, medications used for chronic artery diseases or heart failure; CVA, cerebrovascular accident; DLD, dyslipidemia; DM, diabetes; DVT, deep vein thrombosis; HTN, hypertension; IHD, ischemic heart disease; LLD, lipid-lowering drugs; PPI, proton pump inhibitor; PUD, peptic ulcer disease; SD, standard deviation.
Most patients (29/30, 96.7%) had at least 1 symptom. The most common clinical complaints were abdominal pain, weight loss, and nausea; the other clinical symptoms and their frequencies are shown in Table 2. The patients were symptomatic for a mean duration of 2.8 months (2.8 ± 2.3) before seeking medical care. The physical examination was insignificant in all patients. However, epigastric tenderness was considerable in 2 individuals (6.6%).
The baseline paraclinical studies showed normal white blood cell (WBC) (5540.6 ± 1726.6 /mm3) and normal platelet count (232.0 ± 76.9 /mm3) but decreased hemoglobin levels (11.3 ± 2.2 mg/dL). The complete laboratory assessment results are noted in Table 2.
| Variables | Yes (%) | No (%) |
|---|---|---|
| Clinical symptoms and signs | ||
| Abdominal pain | 23 (76.7) | 7 (23.3) |
| Weight loss | 16 (53.3) | 14 (46.7) |
| Nausea | 11 (36.7) | 19 (63.3) |
| Vomiting | 10 (33.3) | 20 (66.7) |
| Significant weight loss | 10 (33.3) | 20 (66.7) |
| Anorexia | 9 (30) | 21 (70) |
| Malaise | 4 (13.3) | 26 (86.7) |
| Melena | 4 (13.3) | 26 (86.7) |
| Hematemesis | 2 (6.7) | 28 (93.3) |
| Dysphagia | 2 (6.7) | 28 (93.3) |
| Epigastric tenderness | 2 (6.7) | 28 (93.3) |
| The laboratory data | Mean ± SD | |
| WBC (/mm3) | 5540.6 ± 1726.6 | |
| Hb (g/dL) | 11.3 ± 2.2 | |
| PLT (*1000/mm3) | 232.0 ± 76.9 | |
| Cr (mg/dL) | 0.9 ± 0.2 | |
| Blood group | Frequency (%) | |
| A | 13 (43.3) | |
| B | 5 (16.7) | |
| AB | 2 (6.7) | |
| O | 9 (30) | |
| Rh (+) | 5 (17.2) | |
z Abbreviations: WBC, white blood cells; Hb, hemoglobin; PLT, platelet; Cr, creatinine; SD, standard deviation.
The preoperative endoscopic biopsy revealed adenocarcinoma as the primary diagnosis of all but 1 of the biopsies, carcinoma in situ. Based on more detailed pathologic reports, the most common pathologic subgroups were the signet ring cell, intestinal, and diffused type. Notably, the most reported biopsies exhibited poor differentiation (Table 3).
According to the CT scan and EUS findings, most of the patients (43.3%) were in stage III of the disease at the time of the first visit, followed by stages IV, II, and I, which accounted for 26.7%, 10%, and 3.3% of the patients, respectively (Table 3). Most patients (n = 19) had no other concomitant pathology in the CT scan. Still, among the reported accompanying pathologies, the presence of pulmonary nodules (6/30), ascites (4/30), and serous involvement (2/30) were the most common ones.
| Staging | Frequency (%) |
|---|---|
| I. B | 1 (3.3) |
| II. B | 3 (10.0) |
| III. A | 3 (10.0) |
| III. B | 3 (10.0) |
| III. C | 7 (23.3) |
| IV | 8 (26.7) |
| Not specified | 5 (16.7) |
| Concomitant CT scan findings | |
| Pulmonary nodules | 6 (20) |
| Ascites | 4 (13.3) |
| Serous involvement | 2 (6.7) |
| Peritoneal nodule | 1 (3.3) |
| Hepatic mass | 1 (3.3) |
| Splenic artery involvement | 1 (3.3) |
| None | 15 (50) |
| Histopathology | |
| Signet ring cell | 5 (16.7) |
| Intestinal type | 4 (13.3) |
| Diffused type | 2 (6.7) |
| Carcinoma in situ | 1 (3.3) |
| Adenocarcinoma, not specified | 18 (60) |
| Grade of differentiation | |
| Poor | 8 (26.7) |
| Moderate | 4 (26.7) |
| High | 1 (3.3) |
| Not specified | 17 (56.7) |
All the patients underwent diagnostic-staging laparoscopy, which showed ascites, serous involvement, peritoneal seeding, and adhesions in 40%, 30%, 13.3%, and 10% of patients, respectively. Fourteen patients underwent gastrectomy within a mean of 2.3 ± 0.9 months after the final diagnosis. Total gastrectomy comprised 78.5% of all gastrostomies; the rest underwent subtotal gastrectomy.
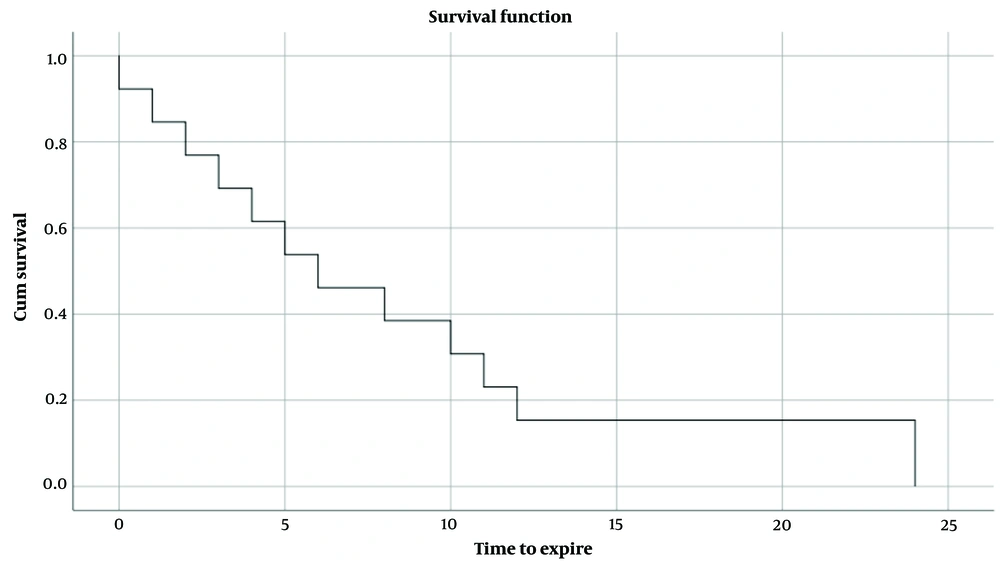
Within the follow-up period, 43.3% (n = 13) of the patients expired. The Kaplan-Meier diagram of the study revealed a mean survival time of 8.4 ± 2.1 months within the 2-year follow-up (Figure 1), with a median of 6.0 ± 2.3 months.
5. Discussion
Our study revealed a male predominance, with a median age of 63.5 years in gastric cancer patients. Most of our patients were in the advanced stages of the disease and became symptomatic less than 3 months before the first visit. Anemia was the most prevalent comorbidity. Surgery was the most applied treatment strategy.
As previously mentioned, gastric cancer is a malignancy of the elderly; most patients are older than 45 years old, and the global median age at the diagnosis is 70 years (7). However, the patients in this study were relatively younger. The median age at the diagnosis was 63.5 years. Previous studies from Iran also have confirmed our results (11-13).
To explain this result, we investigated the prevalence of modifiable and nonmodifiable gastric cancer risk factors among our patients. Smoking, alcohol consumption, and a family history of gastric cancer are among the most notable risk factors in the literature (14-17). In contrast with previous studies, we found no association between these risk factors and gastric cancer. All of them were much lower in our study than in similar articles (18, 19).
Current evidence does not support any predisposing, predictive, or prognostic roles for chronic comorbidities (20-23) that were compatible with our results, as most of our patients had no chronic disease.
While the causative role of H. pylori infection in gastric cancer has been proven, more recent evidence proposes critical roles for other viral diseases such as Epstein-Barr virus (EBV), human papillomavirus (HPV), cytomegalovirus (CMV), and hepatitis B virus (HBV) (24, 25). In a meta-analysis, Li et al. concluded that HBV infection is associated with a poorer prognosis of gastric cancer and can be considered an independent prognostic factor (26). One of the studied patients also had an active hepatitis B infection at the cancer diagnosis. This patient underwent a total gastrectomy and survived until the end of 24 months.
Chronic use of medications also can play an essential role in developing gastric cancer (27). The evidence is limited and controversial, but it seems that PPIs are among the most critical medications that have both protective and harmful effects on the pathogenesis of gastric cancer (28, 29). In our study, most patients also had a history of PPI use, attributable to symptomatic therapy of epigastric pain as the most common clinical complaint.
Almost all evidence agrees that gastric cancer is an insidious malignancy with nonspecific symptoms presenting late in the course of the disease (30). Weight loss, dysphagia, and abdominal pain are the patients’ most common clinical complaints in more advanced disease stages, which align with our findings. Of note, physical examination is unreliable in patients. An extended period with non-specific symptoms leads to the silent progression of the disease, and most patients would present in later stages, negatively impacting the prognosis. Thus, effective screening strategies must be applied in high-risk populations (31-34).
Growing and controversial evidence dedicates prognostic roles to histopathologic features of gastric tumors, which are also sex-dependent (35, 36). We could not assess these claims in our study, but it is highly recommended that further studies be conducted to investigate the difference in tumor histopathology and its prognostic role.
The treatment of gastric cancer is based on timely surgery and applying neo-adjuvant or adjuvant chemoradiation in appropriate cases (37, 38).
With currently available treatment options, the survival rate of gastric cancer is not favorable, and gastric cancer is one of the leading causes of cancer-related mortality worldwide (39, 40). Based on various studies, the 3-year and 5-year overall and disease-free survival rates of gastric cancers are less than 50% (6, 41). According to a review article by Farhood et al., gastric cancer is the deadliest malignancy in Iran (42). In another study, based on a 5-year follow-up of 119 gastric cancer patients, Karimi Jaberi et al. reported a 2-year survival rate of 49.4%, similar to our result (43).
The incidence and prevalence of gastric cancer in North Africa and the Middle East were lower than the global average. According to Ramazani et al., Lebanon, Kuwait, Qatar, Turkey, Oman, Saudi Arabia, and Libya had lower death rates than the incidence rates. However, Morocco, Sudan, Egypt, Yemen, Algeria, Iraq, Palestine, Syria, Jordan, the United Arab Emirates, Bahrain, Afghanistan, Iran, and Tunisia had a higher death rate than the incidence rate (44).
Furthermore, Ramazani et al. (44) reported that disability-adjusted life year (DALY) increased in all countries in the Middle East, except Turkey, from 1990 to 2017, which is higher for men compared with women. The largest increase was related to Iran (48.55%). This can be attributed to the prevalence of H. pylori, smoking, and lack of screening programs for early detection, which seems to confirm our result.
Our study’s most important limitations were single-center inclusion, small sample size, and a relatively short follow-up duration. There were missing data on H. pylori, so it was not included in the final analysis. We suggest further studies be designed as multicenter studies with larger samples and longer follow-ups, focusing on risk factors of gastric cancer, predictive factors of its survival, and its long-term survival.
5.1. Conclusions
After conducting our study on gastric cancer patients, we found that our results confirm the previous findings on the clinicopathologic characteristics of gastric cancer patients. Our study has provided more detailed information on these characteristics. We found that the average age of the patients at diagnosis was younger than expected. Furthermore, the cancer was found to be in advanced stages at the first visit, indicating that early detection and screening are crucial. We also found that comorbidity and risk factors were low among the patients we studied.
Our primary findings include an overall survival rate of 43.3%, indicating that gastric cancer is still a serious health concern. Additionally, during 2 years of follow-up, the mean survival time was found to be 8.4 ± 2.1 months, highlighting the importance of continued monitoring and treatment. Overall, our study provides a more detailed understanding of the clinicopathologic characteristics of gastric cancer patients, which can help inform future research and treatment options.