1. Background
Volleyball is a team sport characterized by explosive movements and maximum exertion, such as power jumps and agile maneuvers, necessitating high levels of physical fitness (1). To achieve peak performance during competitions, elite volleyball players must enhance physiological attributes, including the anabolic state and muscle mass for explosive force generation, in addition to refining skills and techniques (1). With increased intensity in sports like volleyball, the rate of muscle contraction rises, and prolonged activity can disrupt homeostasis, leading to elevated secretion of myokines and cytokines, which are crucial for sports-induced adaptation (1, 2). Achieving optimal physical fitness and effective adaptation to enhance sports performance requires stressing and overloading various body systems (1, 2). Success in many sports is linked to the explosive power of the lower body and the athletes' muscular strength. Moreover, the capacity to generate high levels of strength swiftly (power) is considered vital for optimal performance in volleyball (2). Consequently, choosing the right ergogenic supplements to prepare athletes for improved outcomes and sports skill performance has been a significant concern among coaches and athletes for decades (2, 3).
Leveraging supplements to enhance performance aligns with current trends (3). Beta-alanine recognized as a potential ergogenic aid, is utilized by competitive athletes to boost anabolic hormone production and enhance athletic performance (4). This amino acid, produced endogenously in the liver, can also be obtained from dietary sources like poultry and meat (5). Beta-alanine is essential for carnosine synthesis (6), acting as its rate-limiting precursor. Studies in vitro and in animal models have shown that exogenous Beta-Alanine supplementation significantly increases muscle carnosine concentration, correlating with improved exercise performance (6). Carnosine's role in various physiological processes contributes to exercise capacity and theoretically enhances intracellular buffering capacity (6).
Recent research by Turcu et al. demonstrated that 8 weeks of Beta-Alanine supplementation reduced catabolic responses (CRP) and muscle damage indices (Lactate and CRP), and enhanced anaerobic peak power and performance (4). These findings suggest that Beta-Alanine's contribution to carnosine content modulates catabolic effects and enhances the performance of elite volleyball players (4).
Interleukin-15 (IL-15) has gained attention for its multifaceted role in muscle hypertrophy, prevention of muscle wasting, and its regulatory function on fat mass and energy expenditure (7). IL-15 curbs lipogenesis in white adipose tissue while boosting energy expenditure (8). IL-15, produced during muscle contraction in physical activities, acts as a local factor promoting muscle hypertrophy and stimulating skeletal muscle anabolism (9). It is the most abundant cytokine in skeletal muscle, indicating that muscle activity may significantly regulate its expression and biological functions (9). As an anabolic factor, IL-15 triggers muscle hypertrophy pathways within muscle cells (10). During muscle hypertrophy, IL-15 mRNA is expressed by skeletal muscles, leading to the production and secretion of IL-15 protein, which in turn promotes muscle hypertrophy (11). This cytokine plays a crucial role in the anabolic process by stimulating myocytes and differentiated muscle fibers to accumulate a greater amount of heavy chain contractile proteins (12), highlighting IL-15 as a pivotal mediator for muscle tissue growth and hypertrophy. Consequently, IL-15's activity may be influenced by ergogenic dietary supplements like beta-alanine.
In recent years, irisin and myonectin have emerged as significant myokines secreted in response to physical exercise, as well as dietary glucose and fatty acids, enhancing glucose and fatty acid uptake and oxidation in the liver and adipose tissue (13). Researchers have also found that irisin stimulates browning in subcutaneous adipose tissue, leading to increased thermogenesis and energy expenditure (14). Furthermore, irisin affects skeletal muscle directly (13, 14). Studies have shown that irisin triggers AMPK phosphorylation and glucose uptake in human skeletal muscle cells (15, 16). Myonectin is linked to lipid metabolism, reducing plasma-free fatty acid levels by promoting their uptake in adipose tissue and the liver, thus mitigating plasma FFA concentration (13). A decrease in myonectin levels, which may result from physical inactivity or a high-fat diet, correlates with elevated circulating FFA, accumulation in various tissues, and insulin resistance (15).
Despite the substantial new insights and pleiotropic effects of beta-alanine supplementation, including its connection with IL-15, other myokine signaling, and redox homeostasis, our understanding remains incomplete, with gaps in our knowledge about the downregulation signals responsible for metabolic homeostasis. To further explore this area, we hypothesized that Beta-Alanine supplementation could benefit performance enhancement and metabolic homeostasis.
2. Objectives
We aimed to evaluate the effects of 4 weeks of beta-alanine supplementation on serum myokine levels (IL-15, myonectin, and irisin) and aerobic and anaerobic performance in elite volleyball players aged 22 - 28 years.
3. Methods
3.1. Experimental Approach
This study was a semi-experimental, randomized, double-blind, placebo-controlled trial with a pre-test and post-test design. Eligibility criteria for participation included being a regular volleyball team member, non-smoking, having no history of cardiovascular, blood, liver, kidney, or respiratory diseases, and not using androgenic supplements for at least 3 months prior. At the onset of the study, subjects were required to complete a health questionnaire and consent form and provide personal data. Participants were homogenized based on individual characteristics such as height, age, weight, and body fat percentage. After preliminary arrangements and securing volunteer participation, subjects were incorporated into the study design.
3.2. Subjects
Twenty-two male volleyball players from the Division 1 Volleyball League, aged 22 - 28 years, were selected and randomly divided into two groups of 11 individuals: (1) A supplement group and (2) a control group. The supplement group received 6.4 g/day of Beta-Alanine in 8 servings of 800 mg each through oral administration for 4 weeks, while both groups continued their regular volleyball training three days a week (4, 17), on even days. The control group engaged in the same training regimen but did not consume any supplements.
3.3. Procedures
Before starting the treatment and 4 weeks after, subjects fasted for 12 hours, and 48 hours following the last supplement dose, blood samples were drawn from the anterior axillary vein and centrifuged at 2 500 rpm for 15 minutes at -20°C until biochemical measurements could be performed. The enzyme-linked immunosorbent assay (ELISA) method was employed to determine serum levels of IL-15 (using the VeriKine-HS™ Human IL-15 ELISA Kit, Cat. No. 41702-1), Irisin (using the Cusabio Human Irisin ELISA Kit Catalog Number CSB-EQ027943HU), and myonectin (using the MyBosource Human Myonectin ELISA Kit Cat.No MBS1600042). The Bruce, Rast, and Wingate tests were administered to assess Vo2max, aerobic, and anaerobic capacity, respectively.
3.4. Statistical Analysis
Descriptive statistics were reported using the mean and standard deviation. The Shapiro-Wilk test and Levene’s test were employed to assess the normality and homogeneity of the data variables, respectively. A mixed factorial 2 × 2 analysis of covariance (ANCOVA), with Bonferroni Post hoc analysis, was utilized to evaluate all variables. The covariate was based on the pre-test level variables, while the inter-subject factor was designated for the group. The magnitude of the comparisons between pre- and post-tests for both groups was calculated using Hedge’s g effect size (with a 95% confidence range). Statistical analysis was conducted using SPSS 22 software, with a significance threshold set at P < 0.05.
4. Results
Table 1 illustrates the results of the independent t-test, showing the general characteristics of subjects among the study groups. There was no significant difference between the supplement and control groups in demographic variables such as age (P = 0.95), height (P = 0.54), body mass (P = 0.28), and BMI (P = 0.94), confirming that randomization was effectively applied between the control and supplement groups.
| Parameter | Control Group (n = 11) | Supplement Group (n = 11) | P-Value |
|---|---|---|---|
| Age, year | 24.81 ± 1.32 | 24.45 ± 1.36 | 0.95 |
| Height, cm | 1.84 ± 0.07 | 1.85 ± 0.09 | 0.54 |
| Body mass, kg | 79.63 ± 9.77 | 66.97 ± 9.03 | 0.28 |
| Body mass index, kg/m2 | 22.1 ± 1.6 | 23.8 ± 1.1 | 0.94 |
a Data are expressed as mean ± SD.
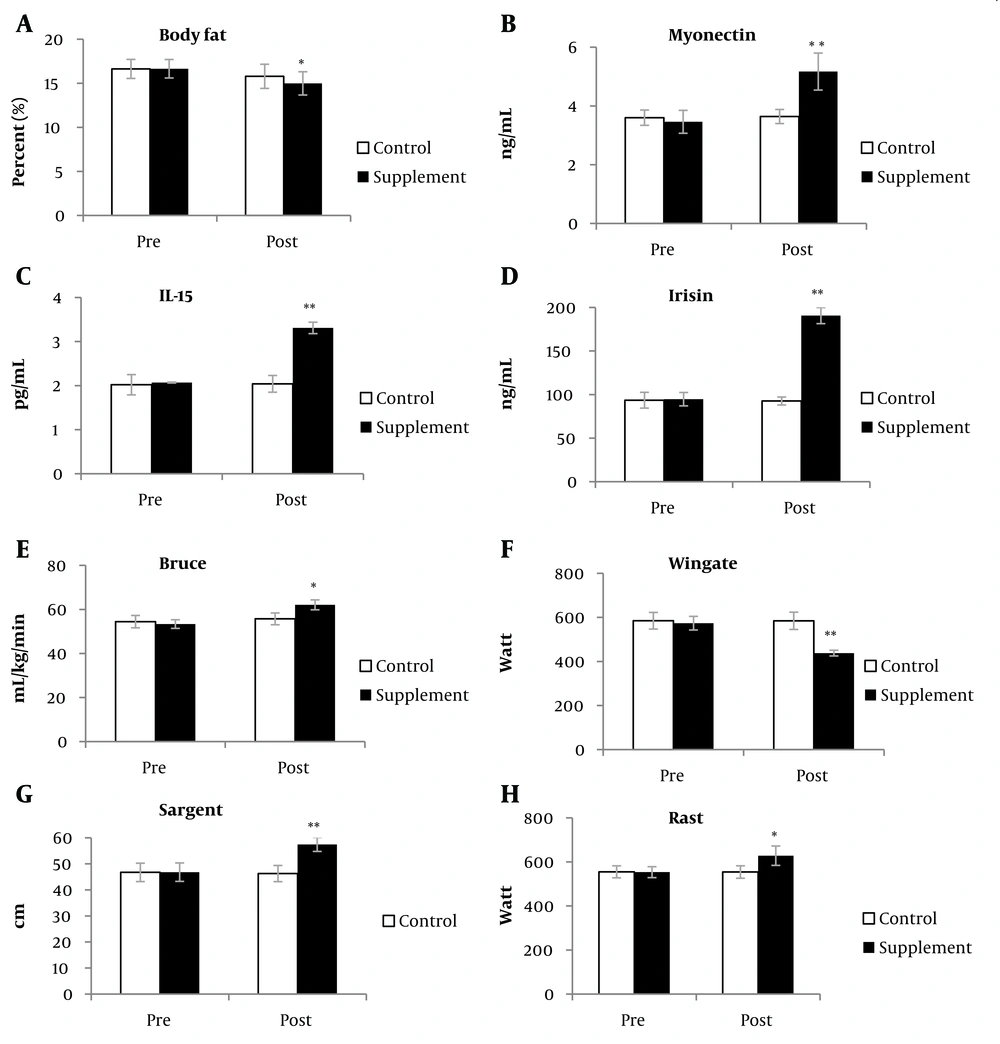
Regarding serum myonectin markers, our analysis indicated that beta-alanine consumption was associated with an increase in serum levels of myonectin (38.63%), IL-15 (60.13%), and irisin (105.05%) in the supplement group compared to the control group. Additionally, records of the Bruce (9.71%), Wingate (23.04%), Sargent (24.22%), and RAST (13.17%) performance tests improved in the supplement group relative to the control group. These results collectively suggest that beta-alanine supplementation enhanced performance skills (Figure 1).
According to the results presented in Table 2, significant differences were observed between the supplement and control groups in the post-test, after adjusting for pre-test scores, in the variables of body fat percentage (mean difference [MD] = -0.93, P = 0.01, confidence interval [CI] = -1.57 to -0.28), myonectin (MD = 1.96, P = 0.001, CI = 1.21 to 2.17), IL-15 (MD = 1.24, P = 0.001, CI = 1.13 to 1.35), Bruce test (MD = 8.66, P = 0.001, CI = 6.68 to 10.65), Sargent's jump (MD = 10.92, P = 0.001, CI = 8.75 to 13.09), and RAST (MD = 75.61, P = 0.001, CI = 54.06 to 97.16). These changes indicate that, compared to the control group, the supplement group showed a lower average body fat percentage and higher averages for myonectin, IL-15, Bruce test, Sargent's jump, and RAST.
| Variable | Group | Mean Difference | P-Value | 95% CI for Difference | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Group × body fat, % | Supplement vs. control | -0.93 | 0.01* | -1.57 | -0.28 |
| Group × myonctin, ng/ml | Supplement vs. control | 1.69 | 0.001** | 1.21 | 2.17 |
| Group × IL_15, pg/ml | Supplement vs. control | 1.24 | 0.001** | 1.13 | 1.35 |
| Group × Bruce, ml/kg/min | Supplement vs. control | 8.66 | 0.001** | 6.68 | 10.65 |
| Group × Sargent, cm | Supplement vs. control | 10.92 | 0.001** | 8.75 | 13.09 |
| Group × Rast, watt | Supplement vs. control | 75.61 | 0.001** | 54.06 | 97.16 |
Linear regression analysis did not yield conclusive results for irisin and Wingate indices; therefore, paired and unpaired t-tests were applied to analyze these data (Table 3). The data in Table 3 reveal that in the supplement group, the paired t-test showed a significant increase in Irisin and Wingate indices (P < 0.01) from the pre-test to the post-test, whereas no significant difference was observed in the control group. Additionally, the unpaired t-test results indicated a significant mean difference between the supplement and control groups for these variables, with the supplement group exhibiting significantly higher serum levels of Irisin and Wingate test scores compared to the control group. This difference was not significant in the pre-test (P < 0.05).
| Variables and Group | Paired Samples t-Test (Mean) | Independent Samples t-Test (Mean) |
|---|---|---|
| Irisin | -95.92** | |
| Control | -1.18 ± 2.69 | |
| Supplement | -98.04 ± 11.95** | |
| Wingate | 135.91** | |
| Control | 10.96 ± 21.38 | |
| Supplement | 146.63 ± 39.48** |
5. Discussion
To the best of our knowledge, this study is the first to explore the effects of beta-alanine supplementation on serum myokine levels and the performance of elite volleyball players over a 4-week study protocol. We hypothesized that beta-alanine supplementation would result in significant performance enhancements. The major findings of this study corroborate our hypothesis, indicating notable improvements in Vo2max, aerobic and anaerobic power, serum myokine levels, and body fat percentage.
5.1. Effects of Βeta-Alanine Supplementation on Body Composition, Physiological Characteristic and Performance
Aerobic and anaerobic capabilities, repeated sprint ability, acceleration, muscular strength, and hypertrophy are identified as the physiological attributes that most distinguish higher-level players from amateurs (18), with muscular strength and power particularly crucial for performance in professional youth volleyball players (1). The observed improvements in jumping performance, aerobic, and anaerobic power relative to the differences in body fat percentage outcomes (P < 0.05) suggest that increases in muscular power production contribute to these performance enhancements and changes in body mass. These findings align with previous research demonstrating significant correlations between body composition and aerobic/anaerobic power in youth volleyball players (19, 20). The level of acidosis induced by a volleyball game appears to be of sufficient magnitude to become a primary performance-limiting factor, causing peripheral fatigue. Thus, intracellular buffers like carnosine are vital for counteracting pH changes and combating fatigue (6, 20). As such, the intramuscular increase in carnosine content due to beta-alanine supplementation may enhance exercise performance across a broad spectrum of high-intensity exercise activities (6, 20).
The 4-week beta-alanine supplementation in this study consistently increased intramuscular carnosine content, which is expected to enhance intracellular buffering capacity. Carnosine's capacity to bind to muscle H+ during intense exercise moderates the decline in intracellular pH, allowing for prolonged exercise duration (6, 21). Higher muscle carnosine levels are known to delay peripheral fatigue onset during intense exercise lasting 10 to 30 minutes and to improve exercise capacity (22). This results in an improved ability to perform better in training sessions by increasing VO2max (22).
Research indicates that the average VO2max value for male volleyball players ranges from 44 to 54 mL/kg/min (23). Although values vary by position, with guards typically having higher aerobic/anaerobic capacity than liberos, Beta-Alanine supplementation in this study increased VO2max from 55.72 ± 2.68 mL/kg/min to 62.09 ± 2.25 mL/kg/min in elite volleyball players (23). Consumption of beta-alanine has been shown to significantly increase carnosine levels in skeletal muscles, correlating with enhanced exercise performance (4, 17). A possible mechanism for these anabolic effects is that carnosine substantially reduces the pro-inflammatory cytokine IL-6, which initiates the downstream inflammatory signaling pathway (4). However, the absence of cytokine measurement represents one of the limitations of the current study. Several studies have demonstrated that Beta-Alanine supplementation induces anabolic effects by down-regulating IL-6, initiating inflammatory responses, and promoting faster recovery after workouts (4).
Recent research has suggested that intramuscular carnosine might also function as a diffusible Ca2+/H+ exchanger at the sarcomere level, enhancing skeletal muscle force production. Carnosine's ability to bind both H+ and Ca2+ suggests that an increase in H+ binding to carnosine may trigger Ca2+ unloading at the sarcomere, leading to an increase in cross-bridge formation and force production. It appears that the enhancement of cellular redox status, resulting in an upregulation of aerobic and anaerobic power, is attributable to this recent mechanism of action (24).
The findings of this study indicated that 4 weeks of beta-alanine supplementation resulted in a reduction of fat weight compared to the control group (P < 0.05). It has been theorized that Beta-Alanine could boost fatty acid metabolism by increasing cellular oxygen consumption and the expression of several cellular proteins linked to enhanced oxidative metabolism. Supporting these results, Schnuck et al. observed an increase in cellular oxygen consumption and the expression of various cellular proteins associated with improved oxidative metabolism in an in vitro setting (20). Furthermore, studies have demonstrated that exercise-induced irisin secretion can modulate muscle metabolism and liver function through AMPK activation, potentially affecting body fat percentage (20).
5.2. Effects of Βeta-Alanine Supplementation on Serum Myokine: IL15, Myonectin, and Irisin
Exercise-induced acidosis has been identified as a causal factor in peripheral fatigue, making intracellular buffers like carnosine crucial for countering pH changes, resisting fatigue, and mitigating subsequent proinflammatory and catabolic responses (25). This study's results revealed that 4 weeks of Beta-Alanine supplementation resulted in increased serum levels of IL-15, Myonectin, and Irisin compared to the placebo group (P < 0.05). Notably, due to the presence of carnosinase in human plasma, direct oral ingestion of carnosine does not increase intramuscular carnosine content. However, intramuscular carnosine levels can be significantly enhanced following beta-alanine supplementation (5). During muscle contraction, skeletal muscle triggers the expression of IL-15 at both mRNA and protein levels in myocytes, leading to hypertrophy through various mechanisms: (1) Accumulation and adhesion of myosin heavy chain filament heads, (2) stimulation of synthesis and inhibition of protein degradation, (3) enhancement of glucose transfer into muscle fibers by increasing the activity of glucose transporters (Glut 4) in the cell membrane, aiding in muscle glycogen storage, and (4) prevention of muscle fiber apoptosis (26).
Unfortunately, this study did not measure the extent of hypertrophy, but the percentage of body fat and total body weight increased following 4 weeks of Beta-Alanine supplementation. These findings lead us to conclude that this myokine acts as an anabolic factor, signaling the muscle hypertrophy pathway in muscle cells, preventing muscle atrophy, and inhibiting the apoptosis of muscle fibers. It also contributes to angiogenesis and facilitates lipid metabolism. Studies have indicated that blocking the IL-15 receptor leads to lipid accumulation in the body, highlighting the critical role of this myokine in preventing free fatty acid deposition in adipose tissue and its potential impact on weight management (10).
Irisin has garnered attention as an "exercise" due to its induction through exercise training in vivo (26). However, the physiology of irisin in humans remains to be fully elucidated. Research, including our own, has documented a significant increase in irisin levels following Beta-Alanine administration. For instance, Al-Sawalha et al. demonstrated the beneficial effects of carnosine on obesity, as well as improved blood pressure and blood glucose levels in rats consuming a high-carbohydrate and high-fat diet (27). Another study by Schaalan et al. found that intraperitoneal administration of carnosine-induced UCP1-positive adipocytes in the white adipose tissues of rats on a high-fat diet and increased plasma levels of irisin, a myokine that promotes beige adipogenesis (28). It has been proposed that muscle mass and capacity play a role in the regulation of basal irisin levels (15). In Schaalan et al.'s study, 6 weeks of Beta-Alanine supplementation (250 mg/kg, i.p.) significantly reduced body weight gain, ameliorated obesity-induced dyslipidemia, decreased thiobarbituric acid reactive species (TBARS) and TNF-α levels, while enhancing serum irisin levels and the expression of adipose uncoupling protein-1 in rats on a high-fat diet (28). Since muscle mass was not measured in the current study, the direct cause-and-effect relationship warrants further investigation. Research has indicated that baseline irisin levels correlate with fitness levels, but ergogenic factors like beta-alanine supplementation could modulate and elevate the circulating levels of this myokine.
Furthermore, the increase in myonectin and IL-15 levels enhances the anabolic effects of beta-alanine supplementation. Conversely, studies exploring the relationship between circulating irisin and metabolic disturbances have identified a negative correlation between catabolic conditions and serum irisin levels, suggesting that myonectin exerts beneficial effects on lipid regulation (13). Petro et al. investigated serum levels of myonectin in adults with metabolic syndrome and reported that myonectin negatively correlates with components relevant to the pathophysiology of metabolic syndrome, such as the android/gynoid fat mass ratio (29). Consistent with our findings, increased levels of IL-15 and irisin were associated with a decrease in body fat percentage in the study subjects. Myonectin levels tend to rise as metabolic conditions improve. Preliminary research indicates that myonectin enhances lipid uptake in adipose tissue and the liver, establishing a potential link between its reduction and impaired lipid metabolism (30). Seldin et al. studied the effects of administering recombinant myonectin to mice and observed reduced circulating levels of free fatty acids. Within skeletal muscle, the soleus muscle (a predominantly slow-twitch, oxidative muscle fiber type) showed a higher expression of myonectin transcript compared to the plantaris muscle (a predominantly fast-twitch, glycolytic muscle fiber type) (31, 32). This suggests that activities involving a high rate of fast-twitch muscle contractions, such as volleyball combined with beta-alanine supplementation, could lead to an upregulation of serum myonectin levels (32, 33).
Thus, considering the results from elite volleyball players, we propose that under anabolic physiological conditions, myonectin modulates lipid turnover through the transport of free fatty acids and the induction of a lipolytic response in central adipose tissue, thereby contributing to the proper accumulation and distribution of fat.
This study faced three primary limitations: (1) The inability to obtain tissue samples prevented us from elucidating biomolecular mechanisms, such as variations in the activities of muscle protein anabolic and catabolic pathways; (2) despite the participants engaging in routine training sessions, we could not measure external workloads or monitor training loads effectively; (3) the assessment of peptides involved in the signaling pathways of anabolic responses represents a third limitation. Future research should investigate the effects of Beta-Alanine supplementation on exercise-induced muscle damage and the biomolecular mechanisms that may facilitate enhanced recovery with beta-alanine supplementation.
5.3. Conclusions
Four weeks of volleyball competition and training resulted in an estimated increase in aerobic and anaerobic power and anabolic myokines in youth professional volleyball players. Beta-alanine supplementation amplified these metabolic and performance improvements. These findings suggest that beta-alanine supplementation could serve as a beneficial nutritional strategy to boost muscular performance. Moreover, in conjunction with increases in the anabolic-to-catabolic ratio observed in this sample, supplementation with beta-alanine could be incorporated into a nutritional plan to enhance volleyball-specific fitness metrics during a competitive season for youth athletes.