1. Background
The 2010 report by the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) indicated that diagnostic radiology accounts for 20% of the total annual effective radiation dose, contributing over 94% to man-made radiation exposure (1). Survey data reveals that in 2016, out of the 235 million people in the US, 24%, including both children and adults under 18, underwent at least one CT scan (2). Notably, one-third of all helical CT scans targeted the head and neck regions (3). Despite the benefits of imaging, ionizing radiation poses a cancer risk, particularly in children, given the sensitive nature of their developing tissues (4). The thyroid gland, being highly sensitive to radiation, is at increased risk during growth phases due to the proliferation of thyroid cells (5). Thus, accurately measuring the radiation dose to the thyroid during imaging, especially in children, is crucial to minimize unnecessary exposure in this vulnerable group.
Various techniques exist for measuring and calculating the organ-specific radiation dose during imaging procedures. One common approach involves using calibrated thermoluminescent dosimeters (TLDs) placed on the skin surface of patients (6). Additionally, experimental methods include employing various dosimeter types or leveraging information from imaging systems to estimate patient doses (7). Nonetheless, these experimental approaches can be costly and time-intensive. Given these challenges, the development of quicker and more precise computational methods is imperative for accurately estimating patient radiation doses.
Monte Carlo simulation offers a streamlined method for assessing radiation doses. This technique models the random behavior of X-ray and radiation interactions with tissues, making it suitable for simulating CT scanners and estimating organ doses (8-10). Unlike traditional methods, it avoids the complications associated with patient data collection and the validation of dosimetry devices (11, 12). Hence, if its precision is confirmed, it can swiftly calculate the radiation dose to patients and identify organs at risk.
2. Objectives
Given the heightened radiation sensitivity in children and the current lack of precise dosimetry data, there is a pressing need to explore quicker and more accurate dose estimation methods for various pediatric age groups. This study focuses on calculating the thyroid dose received during CT imaging in children by employing the Monte Carlo N-particle (MCNP) transport code and comparing these computational results with experimental data gathered from thyroid dosimetry using TLD in patients aged 0 - 5, 6 - 10, 11 - 15, and 16 - 20 years. Leveraging prepared MCNP codes to compute the average patient dose and assess these codes' accuracy will enable physicists to more rapidly estimate the absorbed dose in patients—particularly in vulnerable pediatric groups—using computational methods, thus avoiding the delays and costs associated with traditional dosimetry techniques and preventing unnecessary radiation exposure to patients.
3. Methods
3.1. Monte Carlo N-particle Code
The MCNP code utilizes a Cartesian coordinate system to analyze geometric cells, with each body organ distinctly identified as a separate cell in the simulation. To replicate the human body, two phantoms, one female and one male, from the Oak Ridge National Laboratory (ORNL) were employed (13-15). These phantoms meticulously represent all human body organs, including their materials and densities. However, as these phantoms were originally designed for adult males and females, adjustments were made to the body geometry to accommodate the pediatric age groups of 0 - 5, 6 - 10, and 11 - 15 years, while the same primary phantom configuration was used for the 16 - 20 age group. Specifically, for the ages of 0 - 5, 6 - 10, and 11 - 15 years, average ages of 2.5, 7.5, and 12.5 years were considered. At age 2.5, the geometry of the primary phantom was scaled down to one-fifth (1/5) of the original values, and for the ages of 7.5 and 12.5 years, the dimensions were adjusted to two-fifths (2/5) and three-fifths (3/5) of the primary phantom's size, respectively (13-15). The simulation also defined material cards: Card M1, M2, M3, and M4, corresponding to the materials of air, soft tissue, lung tissue, and bone tissue, respectively (13-15).
3.2. Defining the Source and Simulating the CT Machine
To model the CT scanner (Siemens, SOMATOM Emotion 16 slice, Made in Germany), the simulation included 16 sources to represent the 16-slice device and a plate measuring 22 × 4 cm, arranged equidistantly around a circle with a diameter of 68 cm. While some studies have modeled only the average energy at 120 or 125 kVp (13, 16), others have considered two or three different energy levels (14). In this study, three energy levels of 70, 100, and 140 keV were simulated. It's important to note that the simulation results showed no significant differences across these three energy levels for both 110 and 120 mAs settings, owing to the MCNP code's negligible energy variation at approximately 100 kV and below.
3.3. Output Details
The outputs generated by the MCNP code were normalized per particle exiting the source and needed to be adjusted by the source's power to derive the actual values. The calculations included charges for both electrons and positrons. The standard tallies used were:
- Current passing through a surface (F1),
- Average flux on a surface (F2), and
- Mean flux within a cell (F4).
The total contributions from all particles are reported as the sum of F4. If the
This methodology allows for the calculation of nuclear interactions within a specified cell by incorporating the FMn card after Tally F4 to input the desired volume, cell number, and reaction code.
In this setup, the F4 tally was employed to measure the average photon flux absorbed by the cell (specifically the thyroid).
Two methods are available to translate this flux into a dose. The first involves the use of DEn, DFn, and FMn cards. The DEn card specifies the energy ranges from the source spectrum, which were 70, 100, and 140 keV. The DFn card is responsible for converting absorption and effective flux rates. The FMn card is applied to adjust the code's outcome by the source's power, with the result for a single x-ray then scaled by the flux per second to account for the photon/s device's radiation power using the following formula:
Additionally, the overall irradiation duration for the targeted tissue is another factor that needs to be factored into the final output, yielding the absorbed tissue dose.
Then, 110 and 120 mAs were run for two radiation values to obtain the power of the source, as follows:
Therefore, to put the power of the source in the code, two separate values of 68.75 × 10e + 16 and 75 × 10e + 16 are applied as follows.
FM4 68.75 × 10e + 16
FM4 75 × 10e + 16
Consequently, T4, F4, DEn, DFn, and FMn cards were utilized to compute the dose rate.
3.4. Estimation of Monte Carlo Errors
For a well-behaved calculator, the relative error r would be proportional to N, where N equals the number of stories. In this simulation, the maximum error rate was 0.044, which was acceptable.
Shear reduction variance techniques, such as Time cutoff and Energy cutoff, and methods based on statistical population control, such as Russian roulette, have been used to reduce the statistical error (16).
3.5. Disconnect Cards
The simulation's completion was determined by the required number of particles (n), with the number of particles per second (nps) set at 106. A power cut card was also utilized to eliminate energies below 0.01 eV.
3.6. Russian Roulette
In this design, the Russian roulette method was used to reduce the statistical error. In this regard, the imp photon in each cell should change according to the formula 2n, which would be the opposite:
imp: p 1 2 4 8 16 32 …
The simulation was performed for CTs with 16 slides in rotation and 1s irradiation time.
3.7. Validation of the Code
To validate the accuracy of the simulation code and ensure the CT scan's energy output was accurately replicated, a water phantom measuring 10 × 10 cm was modeled. The absorbed dose within the phantom was calculated at depths of 0 and 5 cm from its surface. Additionally, the actual absorbed dose was measured using TLDs under comparable real-world conditions and subsequently compared with the simulated outcomes (Table 1).
| Simulated Energies and Calculation Method | Depth, cm | |
|---|---|---|
| 1 | 5 | |
| 140 keV and 120 mAs | ||
| TLD dosimetry | 11.9 ± 1.5 | 9.6 ± 1.3 |
| MCNP simulation | 11.3 | 9.2 |
| 140 keV and 110 mAs | ||
| TLD dosimetry | 10.7 ± 1.3 | 8.5 ± 0.9 |
| MCNP simulation | 10.8 | 8.3 |
| 100 keV and 120 mAs | ||
| TLD dosimetry | 8.4 ± 1.6 | 6.3 ± 1.4 |
| MCNP simulation | 8.1 | 6.5 |
| 100 keV and 110 mAs | ||
| TLD dosimetry | 7.9 ± 0.9 | 6.1 ± 1.1 |
| MCNP simulation | 7.1 | 6 |
| 70 keV and 120 mAs | ||
| TLD dosimetry | 6.5 ± 1.6 | 5.1 ± 1.3 |
| MCNP simulation | 6.4 | 5.2 |
| 70 keV and 120 mAs | ||
| TLD dosimetry | 6.2 ± 1.1 | 4.8 ± 0.9 |
| MCNP simulation | 5.8 | 4.3 |
3.8. Experimental Measurements
A case-control study was conducted in the CT scan unit of Be'sat Educational and Medical Center affiliated with Hamadan University of Medical Sciences over a period of six months. The study sample included 45 children and adolescents below 20 years who had undergone brain spiral CT scans. To measure the thyroid absorption dose, GR-200 type TLD tablets were utilized. For calibration, 50 GR-200 tablets were calibrated with a 137-caliber Cesium source and read using a TLD 7103 reader. Calibration factors, both individual and collective, were determined for these tablets (17-19). Detailed information about TLD calibration and the calibration curves is provided in reference (20).
Of the 45 patients analyzed, 27 were in the age group of 0 - 5, 8 were aged 6 - 10, 4 were aged 11 - 15, and 6 were aged 16 - 20. Further details on the parameters used for the x-ray tube, such as average kVp, mAs, field size, slice thickness, pitch, and rotation time, as well as patient dosimetry, can be found in our preceding article (21).
The equivalent dose (ED) was calculated by multiplying the absorbed dose by the radiation weighting factor, set at 1 for X-rays within the CT scan energy spectrum. The effective dose was then determined by multiplying the equivalent dose by the thyroid tissue's weighting factor, which is 0.04 for the thyroid (22).
3.9. Data Analysis Methods
Data were processed using SPSS 16 software, employing central and dispersion statistical measures. The Student t-test (Mann-Whitney test) was applied to assess mean differences between groups.
4. Results
4.1. Simulation Results
The human body simulation was conducted using the MCNP code, employing both male and female phantoms that accurately represented all organs, parts, materials, and densities of the human body. This simulation was performed for a CT device with 16 slices, incorporating rotation and a 1-second irradiation duration. The power of the X-ray source was calculated for outputs of 110 and 120 mAs, resulting in each segment of the patient's head being irradiated for approximately 0.0625 seconds.
The absorption and effective dose of the thyroid gland for the age groups of 0 - 5, 6 - 10, 11 - 15, and 16 - 20 years, for both 110 and 120 mAs settings, are detailed in Tables 2 and 3.
| Age Group, Year and Gender | Absorbed Dose of Thyroid, mGy | Effective Dose of Thyroid, mSv | Exposure Time, Second | Millis Ampere Second, mAs |
|---|---|---|---|---|
| 0 - 5 | 1 | 120 | ||
| Male | 4.165 ± 0.028 | 0.092 ± 0.028 | ||
| Female | 4.185 ± 0.024 | 0.096 ± 0.024 | ||
| 6 - 10 | 1 | 120 | ||
| Male | 3.802 ± 0.036 | 0.079 ± 0.036 | ||
| Female | 3.852 ± 0.044 | 0.086 ± 0.044 | ||
| 11 - 15 | 1 | 120 | ||
| Male | 3.861 ± 0.031 | 0.081 ± 0.031 | ||
| Female | 4.052 ± 0.028 | 0.083 ± 0.028 | ||
| 16 - 20 | 1 | 120 | ||
| Male | 3.021 ± 0.029 | 0.075 ± 0.029 | ||
| Female | 4.072 ± 0.047 | 0.078 ± 0.047 |
| Age Group, Year and Gender | Absorbed Dose of Thyroid, mGy | Effective Dose of Thyroid, mSv | Exposure Time, Second | Millis Ampere Second, mAs |
|---|---|---|---|---|
| 0 - 5 | 1 | 110 | ||
| Male | 3.711 ± 0.037 | 0.081 ± 0.037 | ||
| Female | 3.882 ± 0.040 | 0.086 ± 0.040 | ||
| 6 - 10 | 1 | 110 | ||
| Male | 3.660 ± 0.026 | 0.069 ± 0.026 | ||
| Female | 3.842 ± 0.032 | 0.078 ± 0.032 | ||
| 11 - 15 | 1 | 110 | ||
| Male | 3.252 ± 0.019 | 0.064 ± 0.019 | ||
| Female | 3.412 ± 0.026 | 0.076 ± 0.026 | ||
| 16 - 20 | 1 | 110 | ||
| Male | 3.204 ± 0.031 | 0.060 ± 0.031 | ||
| Female | 3.619 ± 0.029 | 0.065 ± 0.029 |
4.2. Experimental Results of Patients' Dose Measurements
Of the 45 patients analyzed, 31 (52.2%) were boys and 14 (45.2%) were girls, 27, 8, 4, and 6 were in the age groups of 0 - 5, 6 - 10, 11 - 15, and 16 - 20, respectively. For the 11 - 15 age group, there was no imaging of the girl during the study period. The outcomes of the experimental measurements are presented in Table 4.
| Age Group, Year and Gender | Average Age | Absorbed Dose of Thyroid, mGy | Effective Dose of Thyroid, mSv |
|---|---|---|---|
| 0 - 5 | |||
| Male | 3.73 ± 1.334 | 4.853 ± 1.168 | 0.194 ± 0.046 |
| Female | 2.32 ± 1.558 | 6.642 ± 0.418 | 0.226 ± 0.097 |
| 6 - 10 | |||
| Male | 6.714 ± 0.756 | 4.618 ±0.386 | 0.185 ± 0.015 |
| Female | 7.00 ± 0.000 | 5.020 ± 0.000 | 0.201 ± 0.000 |
| 11 - 15 | |||
| Male | 12.5 ± 1.291 | 4.017 ± 0.535 | 0.168 ± 0.021 |
| 16 - 20 | |||
| Male | 16.6 ± 0.548 | 3.954 ± 0.393 | 0.158 ± 0.016 |
| Female | 19.00 ± 0.000 | 4.720 ± 0.000 | 0.189 ± 0.000 |
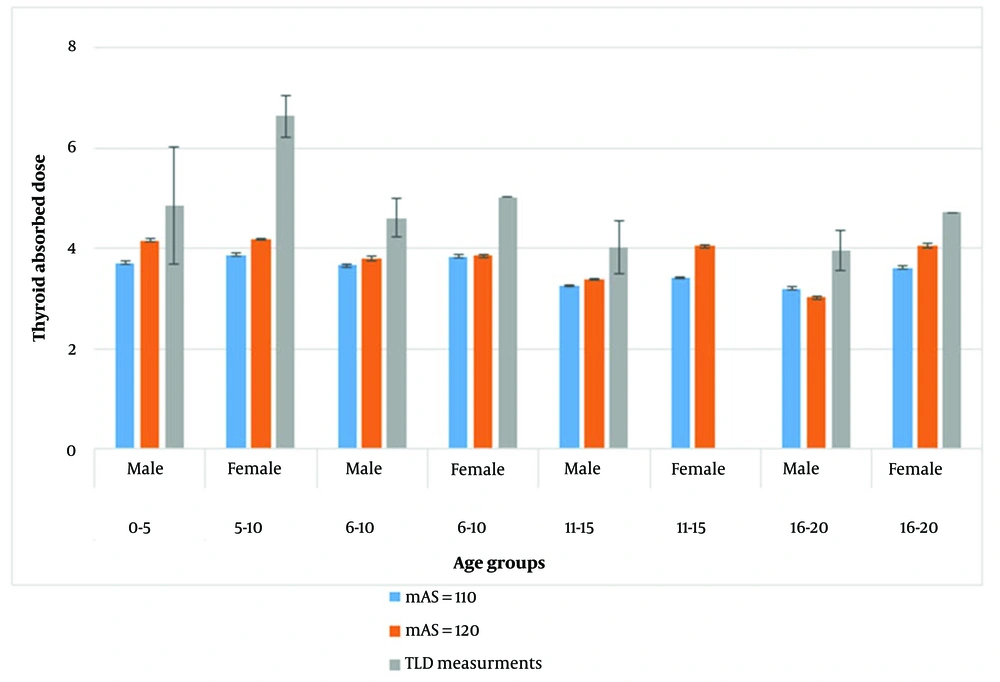
Post hoc test results indicated a significantly higher average thyroid absorption in boys aged 0 - 5 compared to those in the 6 - 10 (P = 0.001), 11 - 15 (P < 0.001), and 16 - 20 (P < 0.001) age groups. Similarly, one-way ANOVA results demonstrated a statistically significant variation in absorbed doses among girls, with post hoc tests showing the average thyroid absorption dose in girls aged 0 - 5 was significantly higher than in the 6 - 10 (P = 0.001), 11 - 15 (P = 0.001), and 16 - 20 (P = 0.001) age groups. However, no significant difference was found in the average thyroid absorption dose between the 6 - 10, 11 - 15, and 16 - 20 year age groups for both boys and girls (P < 0.001). A comparison between the simulated absorption doses and the experimental values is depicted in Figure 2.
This chart illustrates that an increase in mAs significantly raised the thyroid absorption dose across all groups except in the group of 16 to 20-year-old boys. Furthermore, when comparing experimental measurements with simulation results, it was found that the experimental values for all groups exceeded those calculated from the simulation. This discrepancy was significant, with the exception of the 0-5-year-old boys group (P < 0.001).
5. Discussion
In this research, the thyroid absorbed dose for patients across different age brackets (0 - 5, 6 - 10, 11 - 15, and 16 - 20 years) during brain CT scans was calculated using the MCNP code and compared with patient dosimetry results obtained through TLD dosimeters.
MCNP calculations for the thyroid absorbed dose across different age groups revealed that the dose was higher in girls than in boys for both 110 and 120 mAs settings, likely due to differences in the dimensions of the phantoms for girls and boys. At 120 mAs, this gender-based difference was not significant for the first two age groups but was noteworthy for the latter two. For the 110 mAs setting, the difference was not significant across the age groups.
Experimental data collected through TLD dosimetry indicated that the average thyroid absorbed dose for both boys and girls in the 0 - 5 age group was significantly higher than that in other age groups. However, no significant difference was observed among the 6 - 10, 11 - 15, and 16 - 20-year-old age groups.
The thyroid absorbed dose across all age groups and for both genders was higher than the doses predicted by MCNP simulations for both 110 and 120 mAs settings. This variance was not significant for the 0-5-year-old boys' group but was significant for the other groups.
When comparing our findings with other related studies, it is important to note that direct comparison of simulation studies is challenging due to variations in simulation parameters, the structure of the machines, and exposure factors across different research. Additionally, the age groups examined in our study do not fully align with those in other publications. Therefore, we can only discuss and reference articles that share objectives and methodologies similar to our study.
Employing a comparable approach, Li et al. utilized a computer model based on CT data and the Monte Carlo simulation method to assess the organ doses of two patient groups: 5-week-old girls and 12-year-old boys who were subjected to 64-slice CT scans. Their findings demonstrated the feasibility of estimating patient doses using a specifically designed Monte Carlo simulation model. Mirroring the goals of our study, the authors sought to develop a computer program capable of calculating the risk associated with CT scans by determining the average absorbed dose in patients, thereby eliminating the necessity for direct dosimetry (23).
In a related study by Mazonakis et al., conducted in 2007, the thyroid dose received from CT scans across various pediatric age groups was determined using the Monte Carlo code. This approach involved calculating the thyroid dose with mathematical phantoms representing infants and children aged 1, 5, 10, and 15 years, and these calculations were then compared to data gathered from TLD measurements. The study found that the absorbed thyroid dose varied from 0.6 to 8.7 mGy across different head and neck imaging techniques, dependent on the imaging area, patient age, and method used. The discrepancy between the Monte Carlo calculations and TLD measurements was found to be 11.8% (24). Despite differences in the age groups analyzed between Mazonakis et al.'s study and the present one, the thyroid-absorbed doses in comparable age groups align with our simulation outcomes. Thus, these studies collectively suggest that MCNP models can effectively estimate patients' absorbed doses, offering an alternative to direct dosimetry.
Jarry et al., in 2003, conducted a study with objectives similar to ours, aiming to estimate both relative and absolute absorbed radiation doses from axial and spiral CT scans using a Monte Carlo method. They utilized a standard mathematical anthropomorphic model alongside Monte Carlo simulations. Their findings demonstrated that for the head phantom, there was a concordance within 2% between simulated and measured absolute dose data across all slice thicknesses at an energy level of 120 kVp (25).
In a study by Tanyildizi et al. in 2018, the organ doses for both male and female pediatric phantoms across age groups, including newborns 1, 5, 10, and 15 years old, as outlined in the ICRP-89 report, underwent whole-body tomography. The doses were calculated and compared using the Monte Carlo method. The findings were parallel to those of this study, revealing that the thyroid absorbed dose in females was consistently higher than in males across all age groups, though the differences were not statistically significant. Additionally, both male and female pediatric phantoms showed a decline in organ doses with increasing age (26), likely due to the growth in organ volumes and body surface area that comes with age. This observation aligns with numerous other studies in the literature (27-30).
Giansante et al., 2019, conducted a related study measuring lung and thyroid doses during abdominal CT scans in pediatric and adult anthropomorphic phantoms using TLDs, which were then compared to Monte Carlo simulations performed with NCICT. The results indicated that the percentage differences between experimental and Monte Carlo simulated organ doses fell within an interval that was 20% higher compared to the findings of this study (29).
Monte Carlo simulations have suggested that employing a spiral CT protocol for routine procedures could lead to higher thyroid doses than those associated with sequential CT (24), a finding supported by our prior experimental dosimetries using TLDs in both CT modes (21). Moreover, certain studies have pointed out that the increase in scattered dose may be attributed to both z-axis over-scanning and the use of high-pitch values during spiral CT (30). Other research has shown that automatic tube current modulation serves as an effective means to reduce exposure in body regions with non-circular cross-sections, where X-ray beam attenuation significantly varies from one projection to another (31, 32).
This study, along with similar research, indicates discrepancies ranging from 10 to 20% between simulated and experimental dosimetry findings. These variations can be ascribed to uncertainties inherent in TLD dosimeters, statistical errors associated with Monte Carlo simulations, and the discrepancies in size and composition between mathematical models and physical phantoms. Moreover, it's important to highlight that Monte Carlo simulations estimate the mean scattered dose across an area representing the organ, while TLD measurements specifically assess the scattered dose at the organ's central level. Despite these differences, simulating CT scanners and analyzing the thyroid in patients across various age groups is crucial for assessing the average absorbed thyroid dose, particularly in pediatric patients who are more susceptible to the effects of X-ray radiation.
5.1. Conclusions
The findings revealed that the simulated absorbed thyroid dose was consistently higher in girls than in boys. Additionally, experimental data indicated that the average absorbed dose in the thyroid for the 0 - 5 age group was significantly higher than that for other age groups (P < 0.001). Across all age groups, the thyroid absorption dose exceeded the results obtained from MCNP simulations.