1. Background
Nonalcoholic fatty liver is a relatively common condition that includes a wide range of liver damage from simple steatosis or steatosis with mild inflammation of liver cells to severe nonalcoholic steatohepatitis (1). The importance of this disease is due to the destruction of liver cells, and in case of a lack of early diagnosis and proper treatment, it can lead to advanced and irreversible liver diseases such as hepatitis and cancer (2). Nonalcoholic fatty liver disease can be associated with type 2 diabetes, and this combination leads to adverse outcomes of cardiovascular events, which indicates the need for lifestyle modification in the management of nonalcoholic fatty liver disease regardless of diabetes (3, 4). To date, there is no useful and effective drug therapy for non-alcoholic fatty liver disease (NAFLD) (5). Lifestyle interventions, including the Mediterranean diet and increased physical activity, are commonly recommended for the management of nonalcoholic fatty liver disease, as they lead to significant reductions in waist circumference, visceral fat, and blood pressure (6). Diabetes medications, such as metformin, are useful in the treatment of nonalcoholic fatty liver disease because of their multiorgan effects that lead to reductions in plasma glucose and free fatty acids (7, 8). However, clinical trials that have been conducted in recent years have not shown convincing evidence of the usefulness of metformin in improving non-alcoholic fatty liver disease (9, 10). New diabetes drugs, including glucagon-like peptide-1 receptor agonists, sodium-glucose transporter protein-2 (SGLT2) inhibitors, and dipeptidyl peptidase-4 inhibitors (DPP4i), are promising treatment options for patients with type 2 diabetes and nonalcoholic fatty liver disease (11). These diabetes medications have several potentially beneficial effects on glucose and lipid metabolism, including improving insulin sensitivity in hepatocytes and adipose tissue, reducing free fatty acid concentrations, and reducing liver fat content (12).
2. Objectives
Research indicates that to date, few studies have explored the effects of new diabetes medications on NAFLD. Consequently, there is no specific treatment regimen established for using these drugs in treating NAFLD, and the effectiveness and superiority of various drug groups have not been compared to one another. Therefore, this study was conducted to investigate the effect of empagliflozin in combination with pioglitazone on the progression of fatty liver disease in patients with type 2 diabetes who were referred to Imam Reza Hospital.
3. Methods
In this clinical trial study (IRCT20240208060939N1), patients with type 2 diabetes mellitus (T2DM), aged 20 to 70 years, and a hemoglobin A1c (HbA1c) level ranging from 6.5 to 10 were evaluated for inclusion at the endocrinology clinic of Imam Reza Hospital. Eligible patients had both T2DM and NAFLD as diagnosed by a liver ultrasound, with a fatty liver grade of 1 or above.
Exclusion criteria for the study included having type 1 diabetes, active or chronic hepatitis, cirrhosis, biliary disease, class III and IV heart failure, renal dysfunction with a GFR under 45, and a history of alcohol consumption exceeding 20 grams per day for women and 30 grams per day for men. Additionally, patients were excluded if they had been using nonsteroidal anti-inflammatory drugs, amiodarone, tamoxifen, sodium valproate, corticosteroids, methotrexate, drugs related to fatty liver treatment such as vitamin E, empagliflozin, or pioglitazone in the recent months, or supplements such as vitamin C, zinc, selenium, or antioxidant agents. Other exclusion criteria included a history of cardiovascular events within the last 3 months, pregnancy and breastfeeding, active cancer or a history of cancer treatment within the last 2 years, untreated thyroid disorders, or a body mass index above 40 kg/m2.
Eligible participants were randomly divided using a block randomization method into two groups. The first group, consisting of 20 males and 22 females with an average age of 63.3 ± 7.7, received pioglitazone at doses of 15 to 30 mg daily. The second group, consisting of 22 males and 21 females with an average age of 59.9 ± 9.4, received pioglitazone at the same dosing regimen plus empagliflozin (Gloripa, Abidi Company) at a dose of 10 mg daily for a duration of 24 weeks.
At the beginning of the study and again 24 weeks after the start of the intervention, participants underwent a comprehensive evaluation that included BMI and biochemical tests, such as lipid profile, fasting blood sugar, liver function tests [aspartate aminotransferase (AST), alanine aminotransferase (ALT)], and HbA1c levels. Additionally, liver steatosis and fibrosis indices were assessed using two non-invasive methods: Serology and ultrasound imaging. Specific indices measured included the fibrosis-4 (Fib-4) index, aspartate-to-platelet ratio index (APRI), and NAFLD fibrosis score.
To ensure participant adherence and monitor for any potential complications, follow-ups were conducted monthly via telephone and every three months through face-to-face visits.
Data from the study were analyzed using statistical methods appropriate to the data distribution: Student’s t-test for normally distributed data, Mann-Whitney test for non-parametric data, and chi-square test for categorical variables, all performed using SPSS version 22. A P-value of less than 0.05 was deemed significant, indicating statistically meaningful differences.
The ethical committee of AJA University of Medical Sciences reviewed and approved the study protocol, under approval number IR.AJAUMS.REC.1401.009.
4. Results
In the study, changes in fatty liver grade were observed in 32 (76%) patients treated with pioglitazone and 39 (91%) patients treated with the combination of pioglitazone and empagliflozin. However, this difference was not statistically significant (P = 0.07).
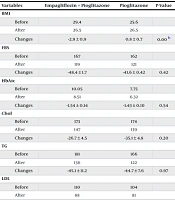
Both treatment regimens improved the indicators of fatty liver, including fasting blood sugar (FBS), HbA1c, cholesterol, triglyceride, low-density lipoprotein (LDL), AST, ALT, NAFLD, and Fib-4 index, with the exceptions of the APRI and the Fib-4 index which did not show improvement. However, no significant differences were observed in the changes of these indices between the two treatment groups, except for high-density lipoprotein (HDL) and NAFLD, where some differences were noted (Table 1).
| Variables | Empagliflozin + Pioglitazone | Pioglitazone | P-Value |
|---|---|---|---|
| BMI | |||
| Before | 29.4 | 25.6 | |
| After | 26.5 | 26.5 | |
| Changes | -2.9 ± 0.9 | 0.8 ± 0.7 | 0.00 b |
| FBS | |||
| Before | 167 | 162 | |
| After | 119 | 121 | |
| Changes | -48.4 ± 1.7 | -41.6 ± 0.42 | 0.42 |
| HbA1c | |||
| Before | 10.05 | 7.75 | |
| After | 8.51 | 6.32 | |
| Changes | -1.54 ± 0.14 | -1.43 ± 0.10 | 0.54 |
| Chol | |||
| Before | 173 | 174 | |
| After | 147 | 139 | |
| Changes | -26.7 ± 4.5 | -35.1 ± 4.8 | 0.20 |
| TG | |||
| Before | 181 | 166 | |
| After | 136 | 122 | |
| Changes | -45.1 ± 11.2 | -44.7 ± 7.6 | 0.97 |
| LDL | |||
| Before | 110 | 104 | |
| After | 88 | 81 | |
| Changes | -22.7 ± 4.5 | -23.2 ± 3.3 | 0.94 |
| HDL | |||
| Before | 38 | 41 | |
| After | 43 | 48 | |
| Changes | 4.6 ± 0.7 | 7.3 ± 1.1 | 0.00 b |
| AST | |||
| Before | 26.9 | 26.8 | |
| After | 20.4 | 19.5 | |
| Changes | -6.4 ± 1.2 | -7.3 ± 1.1 | 0.60 |
| ALT | |||
| Before | 32 | 30 | |
| After | 24 | 20 | |
| Changes | -7.8 ± 2.8 | -9.9 ± 1.3 | 0.58 |
| APRI | |||
| Before | 0.93 | 0.43 | |
| After | 0.68 | 0.25 | |
| Changes | -0.25 ± 0.17 | -0.17 ± 0.04 | 0.15 |
| Fib4 | |||
| Before | 1.34 | 1.33 | |
| After | 1.14 | 1.17 | |
| Changes | -0.19 ± 0.05 | -0.16 ± 0.08 | 0.71 |
| NAFLD | |||
| Before | -0.63 | -0.76 | |
| After | -0.87 | -1.04 | |
| Changes | -0.32 ± 0.08 | -0.24 ± 0.05 | 0.044 b |
a Data are expressed as mean ± SEM and analyzed by Student’s t-test or Mann-Whitney test.
b P < 0.05 was considered as significant.
5. Discussion
An important pathogenic mechanism of NAFLD and type 2 diabetes is insulin resistance. Type 2 diabetes also exacerbates hepatic steatosis, which can progress to fatty liver, fibrosis, and cirrhosis, ultimately increasing the risk of hepatocellular carcinoma (13, 14). In this study, we evaluated two treatment regimens in diabetic patients with fatty liver.
Both intervention groups showed improvements in diabetes indicators, namely FBS and HbA1c levels, aligning with findings from other studies on these drugs (15, 16). However, the comparison between the two groups regarding changes in FBS and HbA1c revealed no significant differences.
A significant reduction in liver enzymes was observed in both groups. Other studies have noted the beneficial effect of empagliflozin on liver enzymes in diabetic patients (17, 18). Specifically, empagliflozin was found to improve fatty liver grades; in our study, 39 participants (91%) in the empagliflozin group experienced a decrease of at least one grade in fatty liver, which nearly reached statistical significance when compared to the pioglitazone group was close to significance. In the study of Aghamohammadzadeh et al. (19), which investigated the weight, lipid profile, and liver enzymes of patients with diabetes, liver enzymes had a significant decrease, except for AST, which did not decrease significantly. In Chawla et al.'s study (20), the obtained results were different from the results of the present study, and based on their study, pioglitazone did not affect liver enzymes. Of course, in the study of Chawla et al. (20) and Aghamohammadzadeh et al. (19), only diabetic patients were considered, and fatty liver disease was not included in the study. The comparison between the two groups (empagliflozin + pioglitazone & pioglitazone) showed no significant difference in terms of the reduction of liver enzymes.
In the current study, significant reductions in the lipid profiles were observed in both groups, aligning with results from the study by AghaMohammadzadeh et al. (19). Filipova et al. (21) reported that pioglitazone improved HDL and total cholesterol in diabetic patients but did not significantly affect LDL and triglycerides. Conversely, Premji et al. (22) found that sodium-glucose transporter protein-2 (SGLT2) inhibitor drugs increased both LDL and HDL levels while decreasing triglycerides. Similarly, a review by Sanchez-Garcia et al. (23) indicated that SGLT2 inhibitors increased total, LDL, and HDL cholesterol levels but were associated with a decrease in triglyceride levels. Cha et al. (24) also noted that the use of SGLT2 inhibitors for 24 weeks resulted in increased levels of HDL and LDL.
In our study, the combination of empagliflozin and pioglitazone significantly decreased cholesterol, LDL, and triglycerides, while increasing HDL, aligning with other studies regarding HDL but showing contradictions in other aspects of the lipid profile. The observed improvements in lipid profiles could be attributed to the combined effects of pioglitazone and empagliflozin, as other studies have also demonstrated the beneficial effects of pioglitazone on lipid profiles (19, 21). The differences between the two treatment groups in our study were significant only concerning HDL, with the pioglitazone group showing a greater increase in HDL levels.
Furthermore, the group receiving both empagliflozin and pioglitazone experienced a significant decrease in BMI, whereas the group treated with pioglitazone alone saw an increase in BMI, though this increase was not statistically significant. This finding is consistent with the results from Biljin et al. (25), where empagliflozin significantly reduced patients' BMI. Other studies in this area have shown similar outcomes (26). Some studies investigating the effects of pioglitazone on BMI also observed results comparable to those of our study (27), suggesting that the combination of these two drugs may have a synergistic effect on metabolic parameters in diabetic patients with fatty liver disease.
In the group treated with empagliflozin plus pioglitazone, there was no significant decrease in the APRI, whereas the pioglitazone-only group showed a significant reduction in APRI following the intervention. However, the comparison of changes in APRI between the two groups did not reveal any significant differences. This finding aligns with the study by Zhang et al. (27), where empagliflozin did not lead to a significant decrease in APRI. Conversely, the study by Lavynenko et al. (28) showed that a drug regimen including pioglitazone, exenatide, and metformin improved APRI in diabetic patients, similar to findings in Ohki et al.'s study (29) where pioglitazone led to a significant decrease in APRI.
Regarding the Fib-4 index, a significant reduction was observed in the empagliflozin plus pioglitazone group, while the decrease in the pioglitazone-only group was close to significant. The comparison between the two groups regarding changes in Fib-4 was not statistically significant. This result contradicts some studies that did not observe a significant improvement in Fib-4 levels among patients using empagliflozin (27).
The NAFLD Fibrosis score significantly decreased in both groups, but the improvement was significantly greater in the empagliflozin plus pioglitazone group compared to the pioglitazone-only group. This finding is similar to Harrison et al.'s study, where the use of pioglitazone improved the NAFLD fibrosis score (30). These results highlight the potential benefits of combining empagliflozin with pioglitazone in improving liver fibrosis indices in diabetic patients with NAFLD.
5.1. Conclusions
It appears that both pioglitazone alone and pioglitazone combined with empagliflozin improve fatty liver disease in patients with type 2 diabetes. However, the effects of these two treatment regimens were not found to be superior to each other.