1. Background
Knee osteoarthritis (OA) is the most common chronic arthritis worldwide, often resulting in knee stiffness, pain, and functional impairment (1). The etiology of knee OA is multifactorial, with pain arising from both supportive extra-articular and intra-articular structures. It imposes a substantial economic burden due to loss of work time, treatment expenses, disability, and comorbid conditions (2). The Institute of Medicine has identified treatments for knee OA as a top 100 priority for comparative effectiveness research (3).
Despite efforts from various clinical trials, there are limited treatment options for knee OA due to the complexity of chronic OA pain (4). Management of knee OA includes weight loss and strengthening, biochemical interventions (such as knee braces, knee sleeves, and foot orthoses), oral analgesics/anti-inflammatories, disease-modifying osteoarthritis drugs (DMOADs), and intra-articular injections (5). Intra-articular injections are the last non-operative modality available for the treatment of knee OA if other self-management and pharmacological treatments are ineffective (6). Types of injections include corticosteroids, hyaluronic acid (HA), botulinum toxin, ozone, hypertonic saline, and dextrose (7, 8).
Hypertonic saline, administered via intra-articular injection, has been widely used as a "placebo group" in many previous clinical trials. Some well-conducted studies have shown that hypertonic saline has a significant pain relief effect as a stand-alone intra-articular injection (9, 10). Hypertonic saline acts as an analgesic by alleviating nociceptive pain from inflamed tissues, which may include bone, connective tissue, synovium, or a combination of these (9, 11).
2. Objectives
The purpose of this study was to evaluate the efficacy of saline prolotherapy versus dextrose prolotherapy for the treatment of knee OA based on a randomized clinical trial. This clinical trial is the first to assess the therapeutic effect of hypertonic saline rather than the placebo effect.
3. Methods
3.1. Study Design
This study was approved by the Research Ethics Committee of AJA University of Medical Sciences (Tehran, Iran) and registered in the Iranian Registry of Clinical Trials Database (IRCT: IRCT20190309042989N1). The study was conducted as a randomized clinical trial.
3.2. Inclusion Criteria
1) Adult patients diagnosed with mild to moderate or moderate to severe OA, as defined by the Kellgren-Lawrence radiographic criteria for OA assessment.
2) Patients with refractory pain to medical treatment.
3.3. Exclusion Criteria
1) Secondary arthritis or rheumatologic diseases.
2) History of joint replacement or recent intra-articular injections with other agents.
3) History of oral or systemic corticosteroid intake within 2 weeks prior to injection.
4) History of anticoagulation therapy.
5) Body Mass Index (BMI) over 40 kg/m2.
6) Alcohol or opium abuse.
7) Acute onset of pain (less than 2 months).
3.4. Participants
Once patients met the radiologic and clinical criteria for knee OA, they were sequentially contacted and provided written informed consent. The study's purpose and potential risks were explained to the participants. Patients using oral supplements or other medications, including NSAIDs, DMOADs, corticosteroids, and analgesics, were asked to discontinue them. Non-pharmacological modalities, including biomechanical interventions (knee braces, knee sleeves, foot orthoses), laser therapy, knee physiotherapy, and TENS, were also discontinued 48 hours before initiating the injections.
3.5. Randomization
Patients were sequentially assigned to Group 1 or 2 of the study arms using computer-generated software. This group allocation was maintained in a blinded database accessible only to the physical examiner, outcome assessor, and radiologist. The injector was not blinded to the type of administered drugs since the volume of administration differed for dextrose and hypertonic saline. Demographic data including age, sex, body weight, height, and BMI were recorded.
3.6. Interventions
Participants received injections of hypertonic saline and dextrose in randomly assigned groups. We used hypertonic saline and dextrose medications from Shahid Ghazi Pharmaceutical Company. Under sterile conditions, administered by an expert physiatrist, the injections were performed. The method of intra-articular injection (inferomedial, medial, or lateral) depended on the degree of access to the joint space measured by plain radiography.
3.7. Hypertonic Saline Injection
A single intra-articular injection of 5 cc of 5% hypertonic saline solution (the most common injectable type of hypertonic saline in Iran) plus 1 ml of Lidocaine 2% was administered into the painful knee joint.
3.8. Dextrose Injection
The dextrose solution contained bacteriostatic water and dextrose. Over two months, injections of 9 mL of 20% dextrose plus 1 mL of Lidocaine 2% were administered in three sessions (baseline, 1 month, 2 months).
3.9. Post-injection Care
Participants were observed for 20 minutes post-injection, as per previous clinical studies (12, 13). In cases of severe pain, patients were allowed to take 500 mg of Acetaminophen. Physical therapy sessions were not permitted for 6 months after the injection to avoid potential confounding effects on trial results. All participants were instructed to perform strengthening exercises to improve lower extremity performance, quadriceps muscle strength, and aerobic capacity.
3.10. Outcome Measures
The primary outcome measure was clinical manifestations assessed by the composite score of the Western Ontario and McMaster University Arthritis Index (WOMAC). A composite score, weighted from 0 (worst) to 5 (best) for knee-related quality of life, was calculated. Sub-scores for pain, function, and stiffness of each knee joint were also determined. The secondary outcome was knee pain, evaluated by the Visual Analog Scale (VAS) score.
3.11. Statistical Analysis
Data were analyzed using SPSS statistical software (version 20, for Windows; SPSS, Chicago, Illinois, USA). Descriptive statistics were used to describe outcomes and baseline clinical characteristics, with mean value ± standard deviation (SD) shown unless specified. Proportional differences and mean differences between the two groups were evaluated using chi-square, Fisher’s exact test, and independent sample t-tests.
4. Results
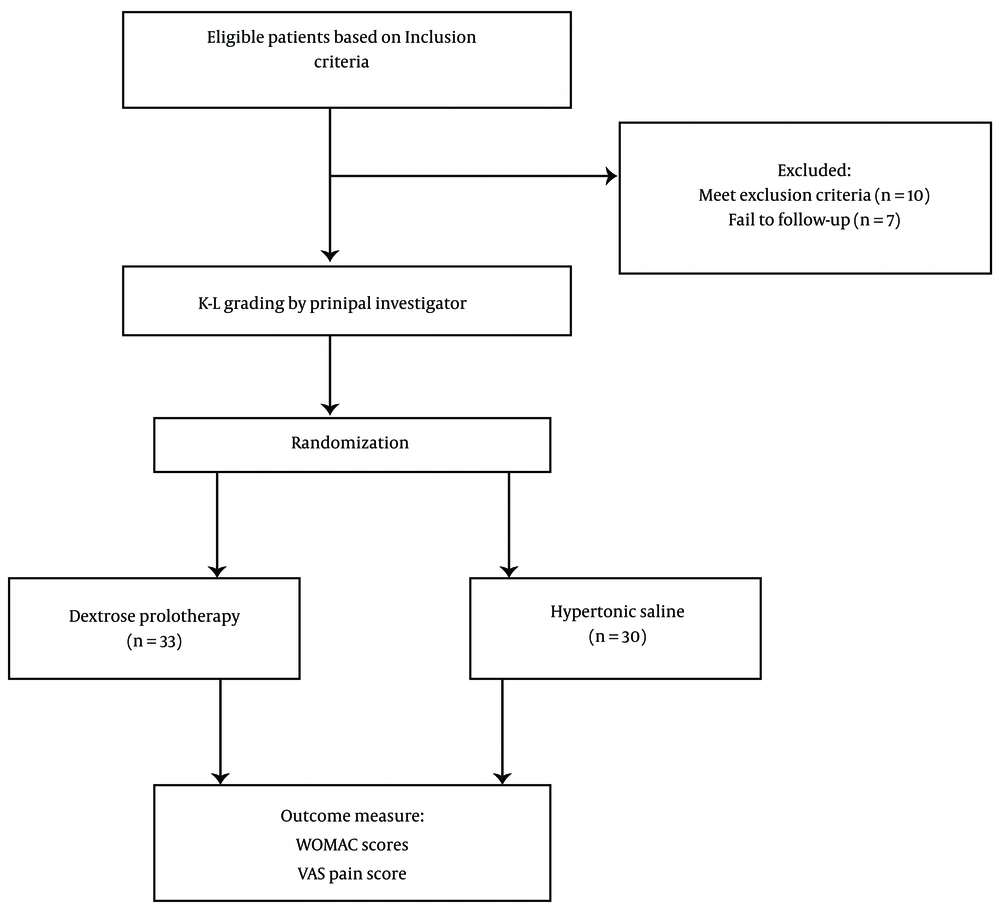
Initially, 80 eligible participants were enrolled in this study, but only 63 patients were able to complete the study period. Seven patients were lost to contact and follow-up, while 10 patients received corticosteroids and other analgesics such as NSAIDs due to reasons like back pain and trauma. Figure 1 illustrates the flow chart of study participants.
Finally, 63 patients completed the 6-month follow-up, with 30 patients allocated to the hypertonic saline group and 33 patients assigned to the dextrose group. Table 1 presents the demographic data at baseline for both study groups. The study sample predominantly consisted of female adults (n = 53, 84.13%), with a mean age of 64.95 ± 9.67 and 47.61% reporting a BMI between 26 and 30 kg/m2. Most participants had tried and failed multiple pharmacological treatments. Radiographic evaluation using Kellgren/Lawrence radiologic measurement showed that the majority of participants (50.79%) had moderate to severe OA (grade 3 or 4 of K-L characteristics) at baseline.
| Variables | Group Saline (n = 30) | Group Dextrose (n = 33) | Total (n = 63) | P-Value |
|---|---|---|---|---|
| Female | 28 (93.33) | 25 (75.75) | 53 (84.13) | 0.090 |
| Age, y | 66.0 ± 10.24 | 63.9 ± 9.11 | 64.95 ± 9.67 | 0.186 |
| BMI, kg/m2 | 0.592 | |||
| ≤ 26 | 4 (13.33) | 5 (15.15) | 9 (14.28) | |
| 26 - 30 | 15 (50) | 15 (45.45) | 30 (47.61) | |
| ≥ 30 | 5 (16.67) | 12 (36.36) | 17 (26.98) | |
| X-ray Klegren-Lawrence osteoarthritis severity score | 0.062 | |||
| 1 - 2 (mild) | 14 (46.67) | 14 (42.42) | 28 (44.44) | |
| 3 - 4 (moderate to severe) | 14 (46.67) | 18 (54.54) | 32 (50.79) | |
| Baseline VAS score | 8.53 ± 1.66 | 7.53 ± 1.56 | 8.03 ± 1.61 | 0.788 |
| Baseline WOMAC score | 2.84 ± 0.44 | 3.04 ± 0.99 | 2.94 ± 0.72 | 0.194 |
a Values are expressed as mean ± SD or No. (%).
There was no significant difference between the two study groups regarding age, gender distribution, BMI classification, and Kellgren/Lawrence radiologic characteristics. Additionally, patients in both the dextrose and hypertonic saline groups showed no significant difference in pain severity measured by VAS (P-value = 0.788) and WOMAC scores (P-value = 0.194) at baseline.
Within-group analysis revealed that in both groups, differences between the outcome scores were significant after 6 months of treatment. Both hypertonic saline and dextrose prolotherapy demonstrated significant improvements in VAS (-4.20 vs. -2.19, respectively), WOMAC composite (-1.23 vs. -1.91, respectively), pain (-1.48 vs. -2.43, respectively), stiffness (-1.01 vs. -0.77, respectively), and function (-1.19 vs. -2.54, respectively), all with P-values less than 0.001. Table 2 describes the within-group comparison of outcomes; Baseline vs. 6 months after treatment.
| Outcome Measure | Mean ± SD at 6 Months | Mean Difference (95% CI) | Within–Group P-Value |
|---|---|---|---|
| Saline | |||
| Visual Numeric Scale | 4.33 ± 2.17 | -4.20 (3.26 – 5.14) | < 0.001 |
| WOMAC composite | 1.31 ± 0.57 | -1.23 (1.06 – 1.39) | < 0.001 |
| Pain | 1.23 ± 0.62 | -1.48 (1.26 – 1.69) | < 0.001 |
| Stiffness | 0.85 ± 0.86 | -1.19 (0.76 – 1.26 ) | < 0.001 |
| Function | 1.85 ± 0.86 | -1.19 ( 0.91 – 1.48) | < 0.001 |
| Dextrose | |||
| Visual Numeric Scale | 4.34 ± 0.28 | -2.19 ( 1.51 – 2.86) | < 0.001 |
| WOMAC composite | 1.33 ± 0.96 | -1.91 (1.62 – 2.20) | < 0.001 |
| Pain | 1.52 ± 1.33 | -2.43 ( 1.99 – 2.86) | < 0.001 |
| Stiffness | 0.69 ± 0.44 | -0.77 ( 0.55 – 0.99) | < 0.001 |
| Function | 1.76 ± 1.39 | -2.54 ( 2.10 – 2.97) | < 0.001 |
Furthermore, Table 3 presents the results of between-group comparison of outcomes at 1, 3, and 6 months after treatment. In the first month, hypertonic saline appeared to have higher VAS scores compared with dextrose (mean difference of 1.56, P-value = 0.006). Also, stiffness decreased more with dextrose rather than hypertonic saline (mean difference of 0.449, P-value = 0.003). The mean WOMAC composite score and pain sub-score showed better outcomes, though non-significant, for hypertonic saline rather than dextrose after 6 months of treatment.
| Variables | Follow-up Stage | ||
|---|---|---|---|
| 1 Month | 3 Months | 6 Months | |
| Visual Numeric Scale | |||
| Mean difference (95% CI) | 1.56 (0.481 - 2.642) | 0.080 (-1.409 - 1.569 | -0.10 (-0.975 - 0.954) |
| P-value | 0.006 | 0.912 | 0.983 |
| WOMAC composite | |||
| Mean difference (95% CI) | 0.04 (-0.363 - 0.450) | 0.028 (-0.343 - 0.399) | -0.017 (-0.416 - 0.382) |
| P-value | 0.827 | 0.879 | 0.933 |
| Pain | |||
| Mean difference (95% CI) | -0.185 (-0.711 - 0.342) | -0.333 (-0.883 - 0.218) | -0.298 (-0.822 - 0.226) |
| P-value | 0.483 | 0.230 | 0.258 |
| Stiffness | |||
| Mean difference (95% CI) | 0.449 (0.162 - 0.736) | 0.411 (0.157 - 0.666) | 0.152 (-0.121 - 0.423) |
| P-value | 0.003 | 0.002 | 0.269 |
| Function | |||
| Mean difference (95% CI) | -0.131 (-0.716 - 0.454) | 0.006 (-0.586 - 0.597) | 0.096 (-0.496 - 0.688) |
| P-value | 0.654 | 0.984 | 0.743 |
5. Discussion
This study unveiled the remarkable therapeutic effects of hypertonic saline on the pain, function, and stiffness of patients with knee OA. Moreover, hypertonic saline prolotherapy yielded similar outcomes in terms of VAS and WOMAC scores after 6 months of treatment compared to dextrose prolotherapy. This is the first study to evaluate the therapeutic effect of hypertonic saline in knee OA rather than relying on the widely known placebo effect.
In our study, we did not report adverse effects after treatment with hypertonic saline and dextrose prolotherapy. However, a previous study by McAlindon reported that while there is no difference in knee pain, 2 years of intra-articular triamcinolone, compared with intra-articular saline, resulted in significantly greater cartilage volume loss and other adverse events (14).
Saltzman et al. designed a meta-analysis to quantify the effect of intra-articular normal saline injections on patient-reported outcomes (PROs). The placebo administration of normal saline yielded a clinically and statistically meaningful improvement in PROs, including VAS pain scores and WOMAC total scores. These observations suggest that the so-called placebo effect for intra-articular normal saline injections yields a meaningful response in patients with osteoarthritis when provided during comparison studies to other active treatment groups such as HA (15).
Recent data introduced dextrose prolotherapy as a cost-effective and meaningful injection-based therapy for chronic pains, including knee OA (16, 17). Dextrose prolotherapy stimulates vascular and fibroblast proliferation, cartilage growth, and dense collagen deposition (18). Moreover, a recent study showed the sensorineural analgesic effect of 5% dextrose prolotherapy in the treatment of chronic low back pain (19). Therefore, the mechanism of action for dextrose prolotherapy is hypothesized to work through reducing peripheral sensitization and targeting structural dysfunction (20).
Rezasoltani et al. designed a randomized clinical trial with four study arms (Physical therapy, intra-articular dextrose prolotherapy, botulinum neurotoxin, and HA). Results showed that dextrose prolotherapy and botulinum toxin type A are effective first-line treatments (21).
Sit et al. tested the efficacy of intra-articular hypertonic dextrose prolotherapy (DPT) vs. normal saline (NS) injection for knee OA in a blinded randomized controlled trial. They showed that intra-articular dextrose prolotherapy injections reduced pain, improved quality of life, and function in patients with knee OA compared with placebo saline injections (20).
The results of the present study elucidated that both hypertonic saline and dextrose prolotherapy are effective in improving clinical symptoms, including function, stiffness, and pain in patients with knee OA; however, dextrose prolotherapy seemed to be more effective in decreasing stiffness in patients. Along with this finding, Lundsgaard et al. reported that distention with physiological hypertonic saline did not significantly improve pain and function in patients with knee OA (22).
Despite some evidence on the effectiveness of saline in knee OA, it seems to be underutilized in the clinic. For future studies, high-quality RCTs are encouraged to describe the efficacy and safety of intra-articular hypertonic saline prolotherapy for knee OA as a therapeutic agent rather than a placebo control. The limitation of our study was that because of the differences in volume administration of dextrose and hypertonic saline, the physician was not completely blinded to the intra-articular injection. Besides, further studies are encouraged with different concentrations and more injection sessions of hypertonic saline in patients with knee OA.
5.1. Conclusions
Among patients presented with knee OA, hypertonic saline prolotherapy performed by a trained specialist results in safe and remarkable outcomes on knee pain, stiffness, and function compared with intra-articular dextrose injection. Our results suggest that hypertonic saline is an effective, cost-effective, and safe choice for patients with mild to moderate knee OA.