1. Context
A significant portion of the global population is infected with Human Cytomegalovirus (HCMV) each year. This large virus is classified as a double-stranded DNA virus, which enables it to persist for years following the initial infection (1). Although HCMV infections in healthy individuals typically present with no symptoms or only mild flu-like symptoms, they can cause severe complications in immunocompromised patients, such as organ transplant recipients or those with acquired immunodeficiency syndrome (AIDS) (2). In recent years, researchers have become increasingly interested in the relationship between HCMV infection and the development or progression of cancer. Numerous studies have investigated the association between HCMV and various malignancies, including prostate cancer (PC), glioblastoma, colorectal cancer (CRC), and breast cancer (BC) (3). While the precise mechanisms by which HCMV may contribute to carcinogenesis remain unclear, the virus has been implicated in promoting cell proliferation, inhibiting apoptosis, and modulating immune responses (4).
During the latency phase, the viral genome persists in a dormant state within the cells, evading the immune system and avoiding detection. However, under certain conditions, such as immunosuppression or inflammation, the virus can reactivate and begin replicating, which in some cases may lead to the development of cancer (5). The presence of HCMV in tumor tissues and the detection of its viral proteins and nucleic acids in cancer cells suggest that the virus may play a role in the development or progression of tumors. Several hypotheses have been proposed to explain how HCMV may influence the development of cancer (6). For instance, HCMV has been shown to modulate cell signaling pathways involved in cell cycle regulation, leading to uncontrolled cell growth and proliferation. Furthermore, the virus can impair DNA repair and regeneration processes, resulting in genomic instability and an increased risk of malignant transformation. Moreover, HCMV has been observed to manipulate the immune system, suppressing immune responses and creating an immunosuppressive microenvironment that facilitates tumor growth and evasion of immune surveillance.
Understanding the role of HCMV in cancer is crucial, as it may have significant implications for developing novel therapeutic strategies (7). Targeting HCMV-related pathways or developing antiviral therapies could offer new opportunities for cancer treatment and prevention. As a result, further research is necessary to fully elucidate the complex interactions between HCMV infection and cancer, as well as the practical implications of these findings. Despite extensive research investigating the association between HCMV infection and carcinogenesis, there are gaps and limitations in our understanding of the precise role of this virus in various types of cancer. These gaps are particularly significant because the increasing prevalence of HCMV in developed societies is a cause for concern.
The objective of the present study is to address these limitations by further exploring HCMV carcinogenesis and the types of cancer associated with it. This study aims to present the mode of infection and carcinogenesis mechanism of HCMV in a more accessible and comprehensible manner, thereby increasing public awareness about this opportunistic virus. Addressing these gaps will contribute to the development of effective HCMV treatment methods and strategies to control the further spread of this viral infection.
2. A Look at Human Cytomegalovirus and the Genome of This Virus
Human Cytomegalovirus, also known as herpes virus type five, has a genome size of 240 kb and a diameter of 150 nm. It is one of 100 beta viruses and encodes between 170 and 750 gene products, with over 40 playing crucial roles in immune response modulation (8-10). Many of these gene products, especially those expressed early in the HCMV life cycle, regulate processes associated with cancer signaling. Human Cytomegalovirus infection can lead to a lifelong presence in humans and other mammals. Approximately 83% of the global population is infected, with the infection rate reaching 100% in developing countries (11). Human Cytomegalovirus can be transmitted through bodily fluids, bone marrow transplantation, and organ transplantation. In immunocompromised individuals, HCMV can cause symptoms such as fever, leukopenia, weakness, and gastrointestinal diseases (12).
3. Pathogenic Process of Human Cytomegalovirus
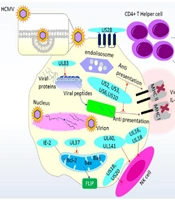
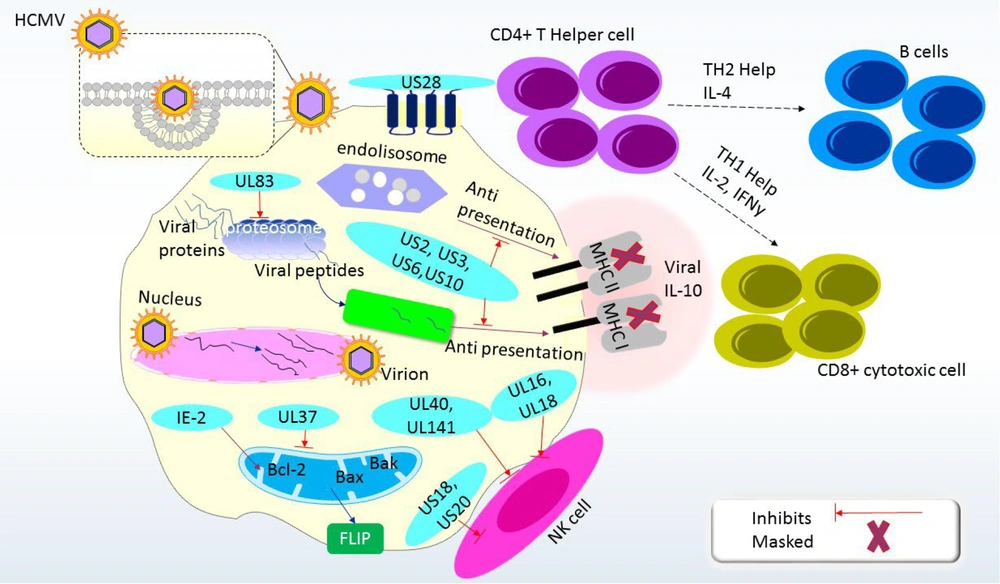
Human Cytomegalovirus can be dangerous for immunocompromised individuals and organ transplant recipients (13). Reactivation occurs when the host cell's immune system is suppressed, and HCMV adapts to the host cell environment through viral transport mediators. Stress can trigger the reactivation of latent HCMV, inducing host immune responses through innate immune processes (12, 14). These responses include inflammatory cytokines and natural killer (NK) cell responses, leading to an adaptive immune response. Human Cytomegalovirus expresses several gene products that alter regulatory proteins, non-coding RNA, and the host immune response (15). To avoid detection, HCMV employs strategies to suppress both the innate and adaptive immune systems (16). Several HCMV gene products, including US20, UL16, UL17, UL18, UL40, UL43, UL140, UL83, UL141, UL144, and UL148, have been identified as important for modulating NK cell activity. The products encoded by the UL16, UL17, UL40, UL140, and UL142 genes interact with the host human leukocyte antigen (HLA) class I antigen to regulate NK cell activity (10). Human Cytomegalovirus downregulates HLA antigen expression and encodes proteins that inhibit peptide presentation by antigen-presenting cells (APCs), limiting T cell responses. It can also inhibit T cell proliferation and upregulate programmed cell death protein 1 (PD-1) receptor expression, causing apoptosis and reducing interleukin (IL)-2 and interferon gamma (IFN)-γ production (14, 17). Tumor necrosis factor α (TNF-α) activates the HCMV immediate-early (IE) promoter in myeloid cells, but on its own, it is insufficient to reactivate latent HCMV. Nuclear factor kappa B (NF-κB) can interact with the HCMV IE promoter, enhancing viral replication. In undifferentiated pre-myelocytic cells, negative regulation of the promoter may allow the virus to persist (9) (Figure 1).
4. Oncogenic Processes Associated with Human Cytomegalovirus
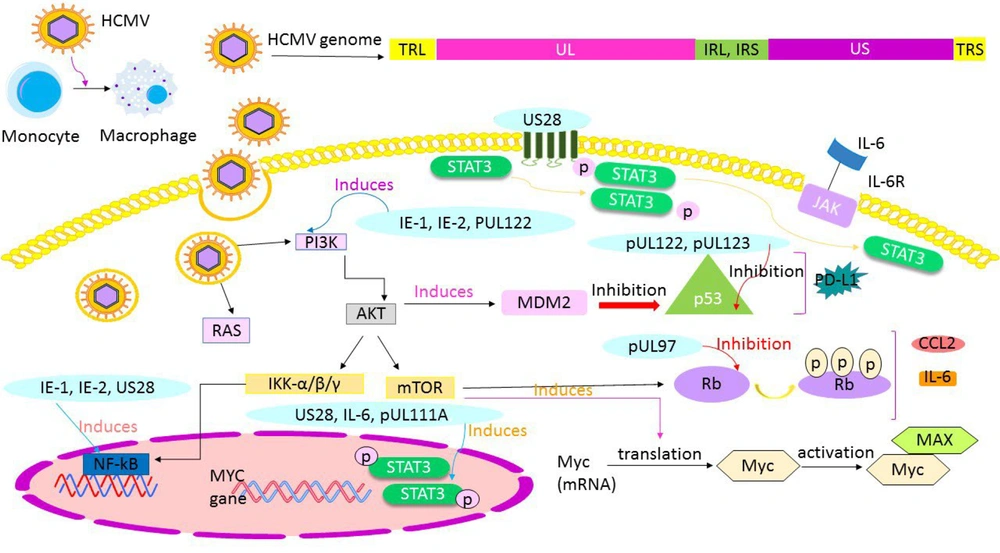
Recent studies have shown that infection with viruses that have high oncogenic potential significantly increases the incidence of cancer. These viruses result from complex host and environmental risk factors that remain poorly understood (18). The relationship between HCMV and cancer has become a critical and necessary topic, with many studies demonstrating the association of HCMV with various types of cancer. The HCMV genome encodes several oncogenes, including US28, IE-1, IE-2, and UL76, which play significant roles in cancer progression and development (19). High-risk HCMV clinical strains, such as HCMV-DB and HCMV-BL, significantly contribute to the development of BC. Human Cytomegalovirus infection often selectively targets tumor cells and specific types of inflammatory cells, rarely spreading to surrounding normal cells (11). Human Cytomegalovirus infection leads to the activation of key signaling pathways associated with cancer, such as IE-1 and US28, which accelerate the oncogenic process in cancer cells (9). High-risk oncogenic strains of HCMV are characterized by increased expression of Myc, activation of the PI3K/Akt pathway, and suppression of p53 and Rb genes (11). The HCMV-induced immunosuppressive factors in the tumor microenvironment include transforming growth factor-β (TGF-β), IL-10, and regulatory T cell (Treg) activation (9) (Figure 2). Additionally, HCMV may play a significant role in cancer development through its ability to cause genomic instability and mutations in the host cell genome (20).
5. Types of Cancer Associated with Human Cytomegalovirus
Several types of cancer have been associated with HCMV. The cancers linked to HCMV infection include those listed in Table 1.
| Some Types of Cancers Associated with HCMV | Carcinogenic Processes Associated with HCMV | References |
|---|---|---|
| HCC | HCMV infection inhibits the activation of the STAT3-Cyclin D1 signaling pathway. By increasing the expression of IL-6, it activates the IL-6R-JAK-STAT3 pathway. | (21-23) |
| BC | Increased overactivation of NF-kB | (24) |
| OC | Increased expression of HCMV primary proteins (IE) and PP65 protein | (25-27) |
| PC | HCMV increases the survival of PC cells due to androgen pathway activation and UL97, which activates the PKA pathway. | (28, 29) |
| Brain tumors | HCMV regulates the expression of the VEGF gene by modulating the activity of the VEGF promoter and activating the NF-κB pathway. | (30-32) |
| GC | Overexpression of UL33 | (33) |
| CRC | Wnt signaling pathway | (3) |
| NB | NB recurs with HCMV infection, and the proteins of this virus are expressed in stem cells. | (34) |
Abbreviations: HCMV, Human Cytomegalovirus; PC, prostate cancer; CRC, colorectal cancer; BC, breast cancer; NB, neuroblastoma; OC, ovarian cancer; HCC, hepatocellular carcinoma; GC, gastric cancer; NF-κB, nuclear factor kappa B.
5.1. Human Cytomegalovirus with Breast Cancer
Breast cancer is one of the leading causes of death among women, affecting approximately 1.5 million people annually (35). Research has shown the presence of HCMV in situ in BC. This virus is found in more than 90% of primary BCs and 90% of metastatic deposits of BC (4). After HCMV-infected monocytes are transferred to breast tissue and differentiate into macrophages, they can facilitate the transmission of the virus to the surrounding breast epithelial cells (36). Most BCs are carcinomas originating in the cells lining the milk ducts of the mammary gland, particularly in altered mammary epithelial cells (37). Furthermore, HCMV found in milk has the ability to directly infect the mammary epithelial cells that line the milk ducts. Over-activation of NF-κB may be caused directly by cytokine production in the tumor microenvironment or indirectly by HCMV infection of breast cells (24).
5.2. Human Cytomegalovirus with Prostate Cancer
Prostate cancer is one of the most common types of tumors, accounting for approximately 350,000 deaths worldwide annually. The two common conditions related to the prostate are benign prostatic hyperplasia and PC, with adenocarcinoma being the most prevalent type of PC (38). While HCMV positivity does not increase the risk of developing PC, it is associated with PC mortality, suggesting that HCMV may have direct effects on tumor cells (39). In healthy prostate epithelium, HCMV infection is often prevalent and promotes PC cell survival and proliferation. Human Cytomegalovirus enhances PC cell survival regardless of androgen receptor status and anti-androgen resistance, partly due to its influence on the androgen signaling pathway and the HCMV UL97 gene (28).
5.3. Human Cytomegalovirus with Brain Tumors
Recent research indicates that human brain tumors (glioblastomas) and pediatric brain tumors (medulloblastomas) frequently contain HCMV proteins and nucleic acids. Glioblastoma is a highly malignant brain tumor that originates from glial cells. HCMV encodes the US28 protein-coupled chemokine receptor, which plays a crucial role in glioblastoma multiforme (GBM) progression (40). US28 regulates vascular endothelial growth factor (VEGF) promoter activity through Gαq (Gq alpha subunit), Gβγ (G beta-gamma complex), p38, and p44/42 kinases, leading to the regulation of the VEGF gene, a key factor in tumor progression in GBM patients (30, 31). In addition to activating oncogenic signaling pathways, causing chromosomal damage, inhibiting cell differentiation, and influencing epigenetic processes, HCMV proteins provide both oncogenic and oncomodulatory mechanisms (41). Human Cytomegalovirus proteins also regulate cell cycle progression by interacting with p53, Rb, and cyclins (42). Furthermore, they stimulate telomerase activity and oncogene expression, cause DNA damage and inhibit DNA repair pathways, and promote inflammation while evading immune system detection (43).
5.4. Human Cytomegalovirus with Colorectal Cancer
Colorectal cancer is a type of cancer that develops in the colon or rectum, the final part of the large intestine. This cancer often arises from damage and alterations in abnormal tissues within the colon wall. CRC is one of the most common cancers, leading to significant mortality (44). Research suggests that HCMV infection may play a potentially significant role in the development and progression of CRC. The results indicate that HCMV often infects or reactivates in the CRC tumor epithelium (45).
5.5. Human Cytomegalovirus with Neuroblastoma
Neuroblastoma (NB) is an embryonic tumor originating from the sympathetic nervous system, which includes the sympathetic ganglia, paraganglia, and adrenal medulla (46). This tumor is recognized as one of the most common and lethal childhood cancers. Recurrence of NB is associated with active HCMV infection. Human Cytomegalovirus proteins are found in NB cells that express the putative stem cell markers cluster of differentiation (CD)133 and CD44. Further research is needed in this area, as the exact mechanisms underlying HCMV's involvement in NB cancer remain unclear (34).
5.6. Human Cytomegalovirus with Rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is a fatal disease characterized by the presence of immature muscle cells, primarily affecting children. The mortality rate for RMS patients is about 50%, and survival rates have not significantly improved over the past few decades. There are three recognized subtypes of RMS: Pleomorphic, alveolar, and embryonal. Children are more likely to develop the embryonal and alveolar subtypes, while adults are more likely to have the more lethal pleomorphic subtype, which has a median survival of 2.25 years (47). Studies have shown that HCMV interacts with several cancer pathways, some of which are associated with RMS (47).
5.7. Human Cytomegalovirus with Ovarian Cancer
Ovarian cancer (OC) is one of the leading causes of death among women worldwide (48). In developed countries, over 90% of malignant OC cases are composed of epithelial cells (49). Recent studies have detected HCMV-glycoprotein B (gB) DNA in 50% of OC cases (50). Research has also shown that patients with OC have higher expression of HCMV-IE and HCMV-pp65 proteins in their ovarian tumors compared to those without OC, even though HCMV infection is rarely associated with OC. Additionally, lower expression of these proteins is linked to reduced tumor survival rates (25).
5.8. Human Cytomegalovirus with Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the most common primary liver cancer (LC), typically developing after years of chronic liver inflammation (51). Human Cytomegalovirus infection prevents the activation of the signal transducer and activator of transcription 3 (STAT3)-cyclin D1 signaling pathway, thereby increasing apoptosis and inhibiting cell proliferation. Additionally, HCMV increases the expression of IL-6, which activates the IL-6R-JAK-STAT3 pathway, leading to increased expression of cyclin D1 and promoting cell survival. These cytokines enhance the growth and proliferation of cancer cells, accelerating the development of HCC (22).
5.9. Human Cytomegalovirus with Muscle Cancer
Muscle cancer (MC) is rare compared to other types of tissue cancers, such as those in the bone, lung, pancreas, and liver. Human Cytomegalovirus typically infects smooth muscle cells, and aortic smooth muscle is often a target for HCMV infection. The presence of CMV DNA has been confirmed through polymerase chain reaction (PCR) test analysis and biopsies of smooth muscle tumors (52).
5.10. Human Cytomegalovirus with Gastric Cancer
Gastric cancer (GC) is the fourth most prevalent cancer globally. Research suggests that HCMV may be associated with several human cancers, including GC. Human Cytomegalovirus encodes approximately 185 genes, with UL133, UL135, and UL136 being linked to GC. UL33 is expressed at significantly higher levels in cancerous tissue compared to normal tissue (33). However, the precise role of HCMV in GC remains largely unknown (1).
6. Ways to Identify and Diagnose Cytomegalovirus Infection
Human Cytomegalovirus is a viral infection that can be identified using various diagnostic methods. The PCR method is the most effective, utilizing genetic techniques to detect the virus in various samples. Immunohistochemistry (IHC) determines the presence of the virus using monoclonal or polyclonal antibodies against HCMV antigens (53). Cell culture is an older method that requires inoculation in fibroblasts and incubation for 2 to 21 days (54). Serological tests, such as CMV IgG or IgM, provide information about a person's previous or new infection by determining the amount of HCMV antibodies in a blood sample (55). Antigen assays, like the pp65 antigen, can be used for rapid diagnosis (56). Saliva and urine testing are preferred for diagnosing CMV infection in infants due to their non-invasive nature and high sensitivity. Healthcare providers can use a combination of these methods to effectively identify and diagnose HCMV infections across different age groups and health conditions (35).
7. Effective Drugs for the Treatment of Human Cytomegalovirus
Human Cytomegalovirus infection is caused by a complex virus that can lead to severe symptoms and is treatable with various medications (57). Ganciclovir (GCV) is the preferred drug for treating HCMV infection and is often administered intravenously. It works by preventing DNA production and inhibiting virus growth and replication. Valganciclovir (VGCV) is a prodrug of GCV that is activated in the intestine and liver, making it suitable for patients who cannot receive intravenous drugs (58). Letermovir (LMV) is an anti-HCMV drug that inhibits the HCMV DNA terminase complex, thereby affecting the production of single-length genomes and virion maturation. Foscarnet (FOS) is a chain inhibitor of DNA phosphorylation, used for GCV-resistant viruses. Cidofovir (CDV) is a nucleoside antiviral drug that inhibits DNA replication and is used to treat serious and resistant HCMV infections, such as HCMV retinitis in HIV-positive patients (59). High-dose prescriptions of valacyclovir, penciclovir, famciclovir, and acyclovir are often given to organ transplant recipients to prevent HCMV infection (60). Maribavir has specific resistance against both HCMV and the Epstein-Barr virus (EBV) but is not effective against other herpes viruses (61). Currently, there is no vaccine available for HCMV infection due to its complexity and the challenges posed by the human immune system (62).
8. Conclusions
The connection between HCMV infection and tumor development has been the focus of recent studies. Cancer is a major global health issue that claims millions of lives annually. Evidence suggests that the HCMV genome contains oncogenes that can contribute to various cancers. However, the exact relationship and mechanisms by which cytomegalovirus contributes to the occurrence and development of cancer have not been fully clarified. More research is needed to investigate this connection and explore methods for controlling and preventing it. Further studies on HCMV and its mode of infection will help identify effective ways to manage and treat diseases caused by this virus. Additionally, by raising awareness about HCMV, its dangers, and prevention methods, we can help prevent the further spread of this virus globally.