1. Background
Polycystic ovary syndrome (PCOS), the most common endocrine disorder in women of reproductive age, affects 8 to 20 percent of women globally who are capable of bearing children (1, 2). Factors such as genetic predisposition, diet, lifestyle, gut dysbiosis, and environmental toxins contribute to hormonal imbalances, including elevated luteinizing and follicular stimulating hormones (LH and FSH), hyperandrogenism (high testosterone levels), and insulin resistance (high insulin levels and low glucose tolerance), all of which characterize PCOS (1). Clinical manifestations such as hirsutism and acne, along with metabolic disturbances and reproductive issues like oligo-anovulation, irregular menstrual cycles, and subfertility, are common in PCOS patients (1).
Metabolic disturbances associated with PCOS can impact other organs, including the brain and liver. Insulin resistance, a hallmark of PCOS, is linked to an increased prevalence of fatty liver disease in affected individuals (3). Additionally, PCOS is regarded as a disorder of the brain, involving dysregulation of the hypothalamic-pituitary-gonadal axis (4).
Black seed (Nigella sativa), known for its therapeutic potential and relatively safe profile (5), contains thymoquinone as its main component, which possesses various beneficial properties such as antifungal, antibacterial, anti-carcinogenic, anti-inflammatory, antioxidant, and anticonvulsant effects (5-8). Investigating whether a dose of 200 mg/kg of black seed extract (BSE) could improve hepatic and brain tissue weight and histopathological markers may open avenues for further research on the therapeutic effects of BSE. Additionally, simultaneous examination of brain and liver tissues in the context of estradiol valerate-induced PCOS and BSE treatment has not been previously explored.
2. Objectives
Given the potential therapeutic benefits of BSE and the documented association between PCOS and metabolic and liver disorders in the literature (3, 9, 10), this study aimed to explore the potential ameliorative effects of black seed hydroethanolic extract on liver and brain tissue weight and histopathological markers in a rat model of polycystic ovary. Given that liver diseases, such as metabolic dysfunction-associated fatty liver disease, can lead to cognitive impairment (11), investigating both liver and brain tissues concurrently was deemed important in this study.
3. Methods
3.1. Polycystic Ovary Model and Study Groups
In this experimental study, 18 female rats aged 2 - 3 months were housed under standard conditions of light (12/12 hours of light/darkness), temperature (22°C), humidity (45 to 55 percent), and diet. The study comprised two phases. In Phase 1, lasting 28 days, the PCO model was induced in all rats by a one-time intraperitoneal injection of 2 mg/kg estradiol valerate (Aburaihan Pharmaceutical Co., Iran) (12). Additionally, during Phase 1, animals in the control group (n = 6) were euthanized 28 days after the estradiol valerate injection. Polycystic ovary syndrome was confirmed through vaginal smear analysis (indicating irregular estrous cycles and the presence of numerous keratinized vaginal epithelial cells) (13) and histological examination (ovarian sections from three rats were stained with hematoxylin and eosin, revealing the presence of cysts) (14) 28 days post-injection. In Phase 2, rats with PCO were divided into two groups: Vehicle (receiving normal saline via gavage for 28 days, n = 6) and BSE (receiving 200 mg/kg hydroethanolic extract of black seed via gavage, n = 6).
3.2. Preparation of Black Seed Extract
Black seeds obtained from a local herb store were authenticated by the Department of Herbal Remedies at Qom University of Medical Sciences (Qom, Iran). Ground black seeds (50 g of powder) were mixed with 200 mL of hydroethanolic solvent (40% water and 60% ethanol) and incubated at room temperature for 72 hours in a sealed container. The solution was then filtered through a Buchner funnel and filter paper size 1. Subsequently, the solution was air-dried at room temperature, yielding a semi-solid mass, which was stored at 4°C until use.
3.3. Tissue Preparation
Prior to sacrifice, rats were anesthetized (using 50 mg/kg Ketamine 10% and 10 mg/kg Xylazine 2%), followed by transcardial perfusion with 20 mL heparinized saline and 4% paraformaldehyde (15). Brain and liver tissues were harvested, washed with saline, and weighed (with connective and adipose tissues around the organs removed). The tissues were then fixed in 10% formaldehyde. After dehydration in ascending grades of ethanol, clearing with xylene, and infiltration with paraffin, serial sections were prepared at 5 mm thickness and stained with hematoxylin and eosin.
3.4. Histological Analysis
Histological analyses were conducted on at least three different rats in each group, with three sections evaluated per animal at considerable intervals (three random fields were assessed in each section). In the liver tissue, the average number of damaged hepatocytes (characterized by segmented nucleus and cytoplasmic shrinkage), Kupffer cells, and lipid droplets were evaluated, while vacuolization, apoptosis, and cell shrinkage were assessed in the brain tissue.
3.5. Statistical Analysis
Normal distribution of data was confirmed using the Kolmogorov-Smirnov test. One-way analysis of variance (ANOVA) with post-hoc Tukey test was performed using SPSS software, version 16 (SPSS, Inc., Chicago, IL, USA) to determine the relationship between groups. Data was presented as mean ± standard deviation, with P ≤ 0.05 considered statistically significant.
4. Results
4.1. Measurement of Brain and Liver Tissue Weight
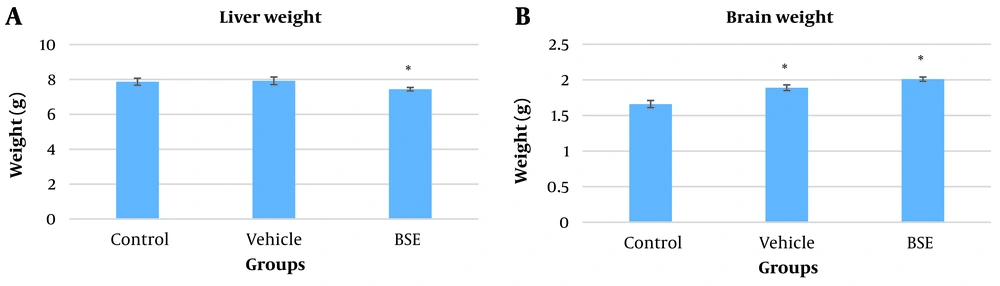
The liver weight in the BSE group (7.44 ± 0.1) was significantly reduced compared to the vehicle (7.92 ± 0.22) and control (7.87 ± 0.2) groups (P ≤ 0.05) (Figure 1A). Conversely, the brain weight significantly increased in the vehicle (1.89 ± 0.04) and BSE (2.01 ± 0.03) groups compared to the control (1.66 ± 0.05) group (P ≤ 0.05) (Figure 1B).
4.2. Damaged Hepatocytes, Kupffer Cells, and Lipid Droplets in the Liver
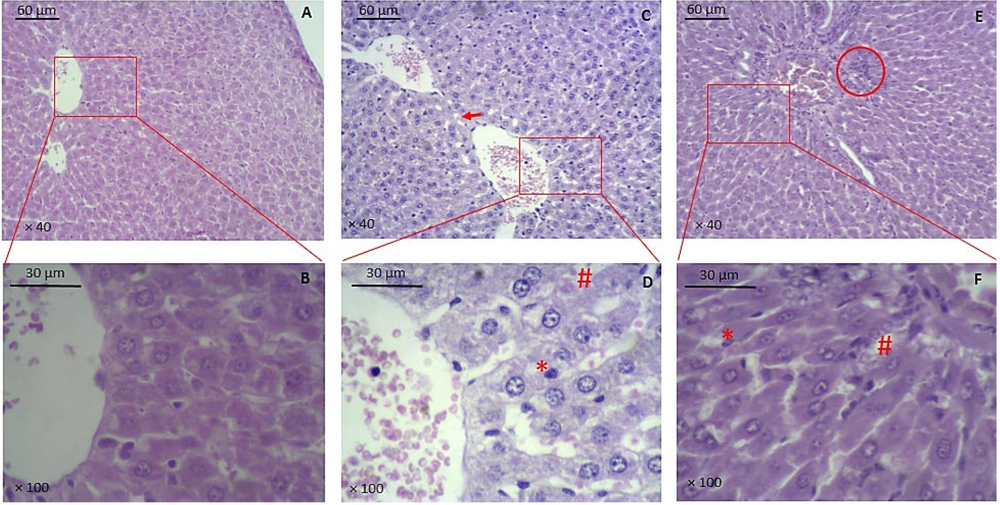
The number of damaged hepatocytes was significantly higher in the vehicle (90 ± 2.5) and BSE (71 ± 1.8) groups compared to the control (25 ± 1.7) group (P ≤ 0.001). Additionally, a significant decrease in the number of damaged hepatocytes was observed in the BSE group compared to the vehicle group (P ≤ 0.05) (Figure 2 and Table 1). There was also a significantly higher number of Kupffer cells in the vehicle (34 ± 1.2) and BSE (24 ± 1.4) groups compared to the control (16 ± 1) group (P ≤ 0.001). Furthermore, a significant decrease in the number of Kupffer cells was observed in the BSE group compared to the vehicle group (P ≤ 0.05) (Figure 2 and Table 1). Changes in the number of hepatocytes with lipid droplets were insignificant between the study groups (Figure 2 and Table 1).
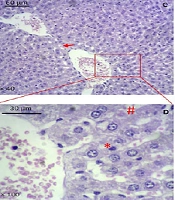
Histopathology of liver tissue. A, B, control group; C, D, vehicle group; E, F, BSE group. * indicates Kupffer cells, # indicates blood cells in sinusoids, arrow indicates vacuolation or lipid droplets, circle indicates cholangitis. (A, C, and E are × 40 magnified, and B, D, and F are × 100 magnified; H&E staining; BSE: Black seed extract).
a Values are expressed as mean ± standard deviation and analyzed by ANOVA and post-hoc Tukey’s test.
b Compared to the controls (P ≤ 0.001).
c Compared to the vehicle (P ≤ 0.05).
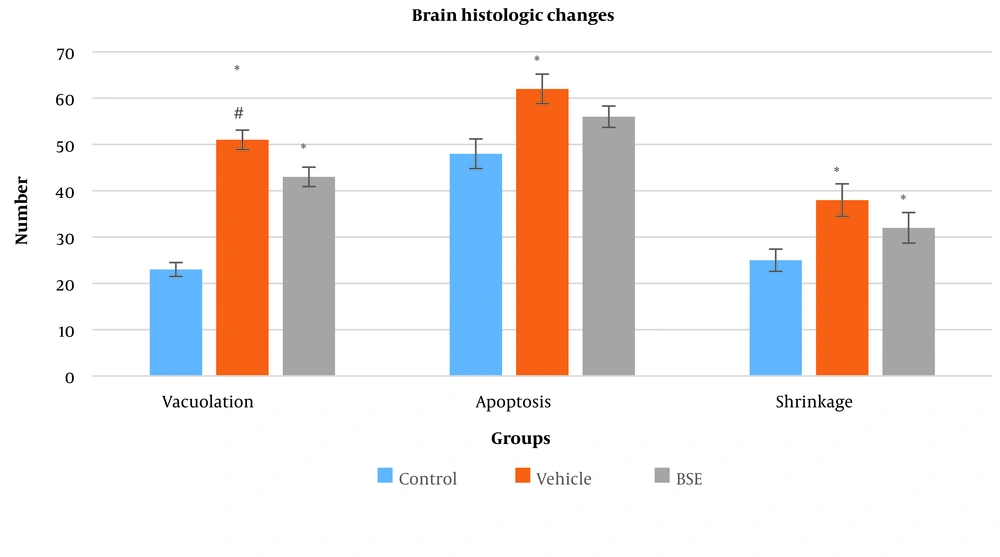
4.3. Vacuolation, Apoptosis, and Shrinkage in the Brain
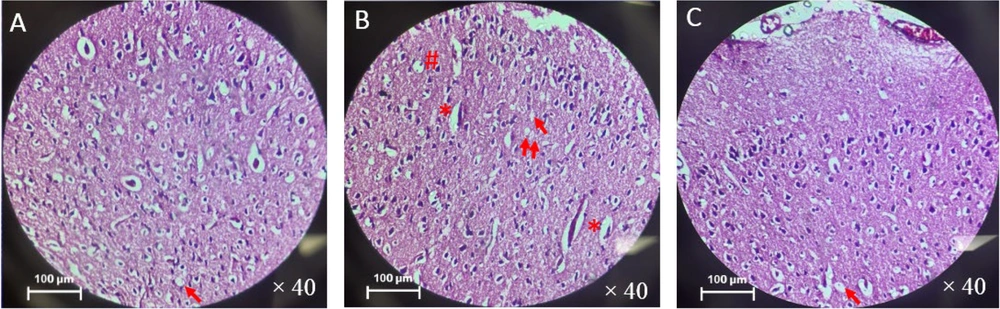
The number of vacuolation in the vehicle (51 ± 2.1) and BSE (43 ± 2.1) groups was significantly higher compared to the control (23 ± 1.5) group (P ≤ 0.001). Additionally, a significant decrease in the number of vacuolations in the BSE group compared to the vehicle group was observed (P ≤ 0.05) (Figure 3). There was also a significantly higher number of apoptotic cells in the vehicle (62 ± 3.2) and BSE (56 ± 2.3) groups compared to the control (48 ± 3.2) group (P ≤ 0.001) (Figure 3). Furthermore, a significant increase in the number of cells with shrinkage in the vehicle (38 ± 3.5) and BSE (32 ± 3.3) groups compared to the control (25 ± 2.4) group was observed (P ≤ 0.05) (Figures 3 and 4).
5. Discussion
This study demonstrated the adverse effects of PCO on liver and brain tissue through the increased number of damaged hepatocytes and Kupffer cells in the liver, and the increased number of vacuolation, apoptosis, and shrinkage in the brain tissue. The possible ameliorating effects of BSE were observed in decreasing the damage in both tissues through histologic evaluation. Furthermore, the liver's weight decreased and the brain's weight increased after BSE treatment.
Following a one-time injection of estradiol valerate, the PCO model was induced in rats. Studies demonstrate the efficiency of this agent in inducing the PCO model (16, 17). Estradiol valerate's mechanism of action includes dysregulation of the hypothalamus-pituitary-ovarian axis and activation of sympathetic innervation to ovaries, which leads to the suppression of ovulation (16, 17).
Increased serum inflammatory factors and an increased number of Kupffer cells and damaged hepatocytes following PCO are reported in several studies (9). Nonalcoholic fatty liver disease can be found in 47% of patients with PCO (10). Increased androgens in PCO lead to increased lipolysis and free fatty acids, which are primary ligands for immune system receptors. Therefore, the higher levels of free fatty acids activate the inflammatory process (10).
Antioxidant and anti-inflammatory impacts of BSE have been reported in several studies (18, 19). Kushwah et al. (20) have reported decreased serum liver-specific enzymes and malondialdehyde and increased antioxidant enzymes in the liver following BSE treatment. Also, improved histopathology of the liver tissue was reported in this study. Protective effects of BSE have also been reported in Abdel-Daim and Ghazy(21) study in which the mechanism of protection is through the suppression of free radicals and antioxidant activity of BSE.
Neuroprotective, memory, and learning-boosting effects of BSE have been reported in several studies (22, 23). Increased weight, better learning, and spatial memory possibly because of oleic acid, linoleic acid, and antioxidant compositions in BSE are reported in previous studies (23). Ferdosi Makan et al. (22) have reported neuroprotective impacts of BSE through increased neuronal density following BSE treatment in alpha motor neuron degeneration in rats undergoing sciatic nerve compression.
Increased volume and weight of the liver in patients with liver inflammation and steatosis are suggested in the literature (24). In this study, the decreased weight of the liver tissue accompanied by improved histopathology in the BSE group can be regarded as a sign of the healing effects of BSE. Furthermore, reduced brain tissue volume and weight are obvious in neurodegenerative diseases or normal aging due to neuronal loss (25, 26), thus the increased weight of the brain tissue accompanied by ameliorated histopathology would show affirmative impacts of BSE in this study.
Liver diseases such as fatty liver disease associated with metabolic dysfunction involve extrahepatic manifestations in the form of cardiovascular, kidney, and cognitive disease (11). Studies suggest that patients at risk of liver fibrosis may show impaired executive function, indicating a potential link between liver and brain diseases, referred to as the liver-brain axis (11, 27, 28). Our study may demonstrate the boosting effects of BSE on liver and brain tissue, suggesting a possible direct neuroprotective effect of BSE or indirect boosting effects through the improvement of liver histopathological markers.
In our previous experience with the effect of BSE on PCO rats, we observed remarkable positive results in serum (increased luteinizing hormone and testosterone) and ovarian tissue (increased number of primary and Graafian follicles and decreased cystic follicles) when higher doses of BSE (200 mg/kg) were administered (29). The neuroprotective effects (increased neuronal density) and antioxidant properties of BSE may be responsible for mild boosting effects on brain tissue by preventing cell death and increasing cell survival (22).
5.1. Conclusions
As the potential therapeutic impacts of BSE are emphasized in the literature and enhanced histopathological evidence in the liver and brain tissue in this study, BSE could ameliorate the adverse impacts of PCO in the liver (reducing damaged hepatocytes and Kupffer cells) and brain (reducing vacuolation). It can be concluded that BSE can be regarded as a potent healing herb in several diseases without side effects. However, further investigation into the potential protective effects of BSE is warranted through additional histological and molecular analyses.