1. Background
Red blood cell distribution width (RDW) is a parameter that indicates the variation in size of red blood cells (RBCs) (1) and is typically reported as part of a standard complete blood count. RDW, along with other parameters from a complete blood count, is used to identify hematological diseases (2). Recently, it has been shown that RDW can also be used to predict mortality in coronary artery diseases, acute heart failure, and acute myocardial infarction (AMI) (3). One study suggested that RDW may be a reliable marker for mortality in both chronic and acute conditions (4). The majority of cardiovascular deaths occur following AMI (5), which is one of the most serious cardiovascular events (2, 6).
MI is a severe, life-threatening event that is associated with an increased risk of anxiety (7). There is a growing association between anxiety and cardiovascular disease (CVD) (8). Independent of CVD, depression and anxiety are major contributors to disability and mortality, affecting 260 million people globally (8). Recent studies have shown that anxiety is associated not only with serum C-reactive protein levels but also with blood inflammatory markers, including white blood cell (WBC) count and RDW (9). Furthermore, several studies have examined blood indices, including RDW, in psychiatric populations. Measurement of these parameters is simple and inexpensive, making it an appropriate choice for frequent assessment (10, 11).
2. Objectives
Given the cost-effectiveness, availability, and routine use of the RDW test (12) and the known relationship between anxiety and CVD, including AMI, we decided to investigate the changes in RDW and its relation to anxiety two weeks after AMI.
3. Methods
3.1. Participants
In this cross-sectional study, 250 consecutive patients with AMI who were referred to Dr. Heshmat Hospital in Rasht, Iran, during 2023-2024 were investigated. The study procedure began after obtaining ethical approval with the code IR.GUMS.REC.1402.237 from Guilan University of Medical Sciences. Patients were included in the study if they had a diagnosis of AMI confirmed by a cardiologist, were over 18 years of age, had adequate cognitive ability to answer questions, and did not have mental or physical problems, including neurological and mental diseases such as depression. The exclusion criteria included the presence of fatigue before the occurrence of AMI (according to the patient’s statements), failure to measure RDW within 24 hours after admission, presence of blood diseases such as leukemia and myelodysplastic syndrome, and death during hospitalization or within one day of discharge. Informed consent was obtained from all patients who participated in the study.
3.2. Data Collection
Demographic information such as age, sex, systolic and diastolic blood pressure (BP), and history of diseases was obtained from patients’ medical records. Laboratory parameters, including fasting blood sugar (FBS), total cholesterol, triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL), creatinine, blood urea nitrogen (BUN), and RDW (our main variable), were collected within 24 hours after admission from the laboratory test results. The intensity of anxiety was evaluated using the Hamilton Anxiety Rating Scale (HRSA) at baseline and two weeks after the occurrence of AMI. The HRSA consists of 14 questions, each scored up to 4 points, with a maximum total score of 56. We considered a total score of less than 25 as indicative of no anxiety and a score of 25 or greater as indicative of anxiety.
3.3. Statistical Analysis
Qualitative variables were reported as frequency and percentage. The Kolmogorov-Smirnov test was used to check the normality of quantitative variables. If these variables were normally distributed, they were reported with the mean and standard deviation; otherwise, they were reported with the median and interquartile range. After collecting the data, based on the results of the HRSA, the data were divided into two groups: With anxiety and without anxiety. The initial investigation of the research variables in these two groups was performed using independent t-tests, Mann-Whitney tests, chi-square tests, or Fisher's exact tests. Finally, to assess the relationship between RDW and anxiety while adjusting for auxiliary variables, a logistic regression model was fitted to the data. Data analysis was performed using SPSS version 24 software.
4. Results
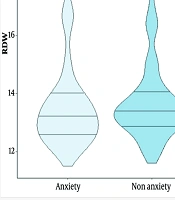
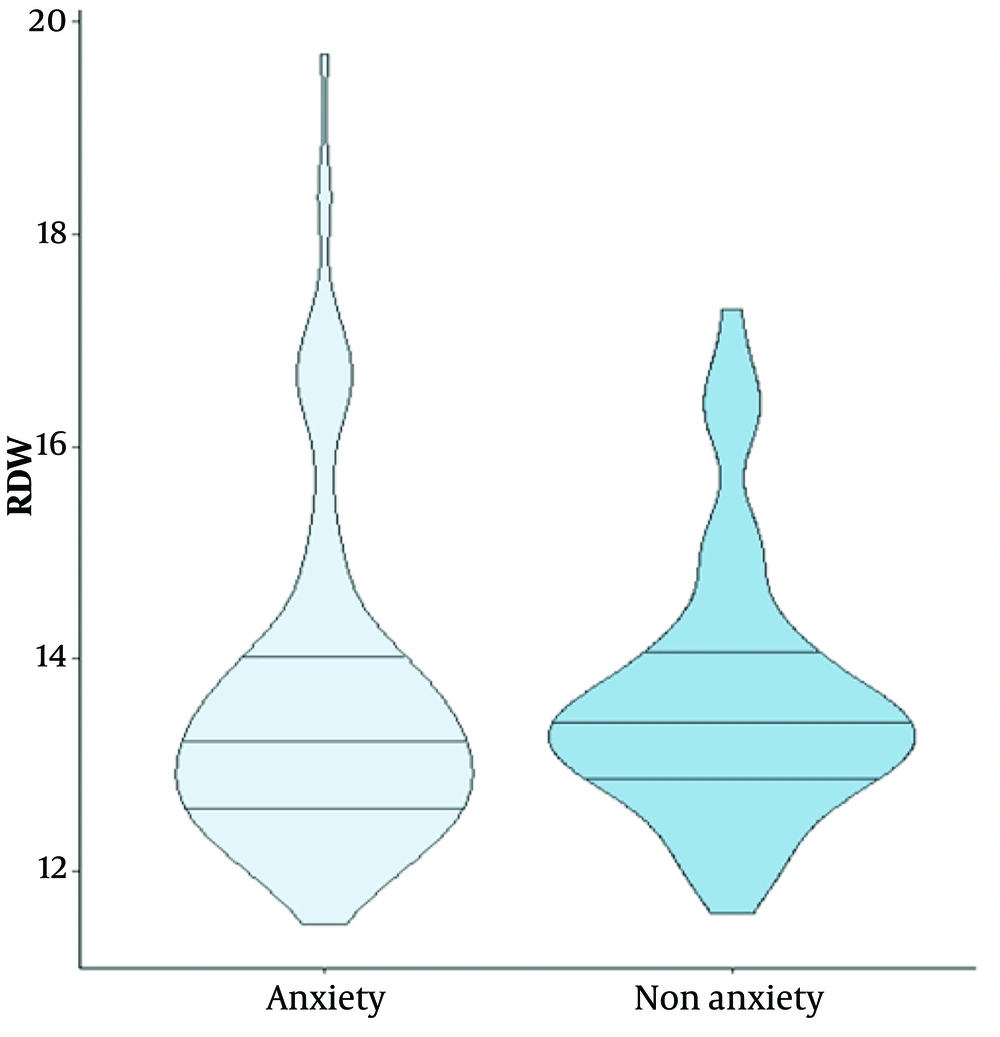
Based on our findings, 129 (51.6%) patients in our study experienced anxiety two weeks after the occurrence of AMI. Of the total 250 participants, 69.8% were male and 30.2% were female. The mean ± SD age of patients in the anxiety and non-anxiety groups was 59.77 ± 10.10 and 62.68 ± 12.20 years, respectively. Among the laboratory parameters, only blood urea nitrogen (BUN) was statistically different between the two groups (P = 0.027). The RDW was 13.63 ± 1.20 in patients with anxiety and 13.59 ± 1.51 in the non-anxiety group (P = 0.174). Other patient information is shown in Table 1.
| Variables | Anxiety 129 (51.6) | Non-anxiety 121 (48.4) | P-Value |
|---|---|---|---|
| Gender | |||
| Male | 90 (69.8) | 92 (76) | 0.266 |
| Female | 39 (30.2) | 29 (24) | |
| Age | 59.77 ± 10.10 | 62.68 ± 12.20 | 0.040 b |
| Laboratory parameters | |||
| FBS | 137.99 ± 62.52 | 138.65 ± 64.34 | 0.932 |
| Chol | 155.71 ± 52.91 | 152.65 ± 44.14 | 0.971 |
| TG | 202.92 ± 150.21 | 189.69 ± 146.6 | 0.152 |
| BUN | 19.85 ± 6.76 | 18.38 ± 6.05 | 0.027 b |
| Cr | 1.12 ± 0.37 | 2.3 ± 10.66 | 0.061 |
| RDW | 13.63 ± 1.20 | 13.59 ± 1.51 | 0.174 |
| Underlying diseases | |||
| HTN | 77 (59.7) | 66 (54.5) | 0.411 |
| HLP | 57 (44.2) | 53 (43.8) | 0.951 |
| DM | 39 (30.2) | 49 (40.5) | 0.090 |
| Arthritis | 1 (0.8) | 3 (2.5) | 0.357 |
| CVDs | |||
| Previous | 11 (8.5) | 3 (2.5) | 0.038 |
| AMI | 6 (4.7) | 0 (0) | 0.30 b |
| Systolic BP | 134.34 ± 20.04 | 136.72 ± 22.31 | 0.274 |
| Diastolic BP | 79.51 ± 17.21 | 79.92 ± 11.08 | 0.659 |
Abbreviations: AMI, acute myocardial infraction; BP, blood pressure; Bun, blood urea nitrogen; Cr, creatinine; Chol, cholesterol; CVDs, cardiovascular diseases; DM, diabetes mellitus; FBS, fasting blood sugar; RDW, red blood cell distribution width; TG, triglyceride; RDW, red blood cell distribution width.
a Values are expressed as mean ± SD or No. (%).
b Significant.
Figure 1 shows the violin plot for RDW distribution in the anxiety and non-anxiety groups. The median and the first and third quartiles are nearly identical in both groups. The violin plot also identifies an outlier in the anxiety group.
Multivariable regression was used to assess the relationship between RDW and anxiety. In model 1 (unadjusted model) [OR (odds ratio), 0.978; 95% CI, 0.816 - 1.173; P = 0.809], model 2 (adjusted for age and gender) [OR, 0.960; 95% CI, 0.798 - 1.154; P = 0.661], model 3 (adjusted for age, gender, and BUN) [OR, 0.949; 95% CI, 0.787 - 1.144; P = 0.584], and model 4 (adjusted for age, gender, BUN, previous AMI, and CVD) [OR, 0.932; 95% CI, 0.769 - 1.130; P = 0.475], no significant relationship was found between RDW and anxiety (Table 2).
| Model | SE | Wald | OR (95%CI) | P-Value |
|---|---|---|---|---|
| Model 1 | 0.093 | 0.058 | 0.978 (0.816 - 1.173) | 0.978 |
| Model 2 | 0.094 | 0.192 | 0.960 (0.798 - 1.154) | 0.661 |
| Model 3 | 0.095 | 0.299 | 0.949 (0.787 - 1.144) | 0.584 |
| Model 4 | 0.098 | 0.511 | 0.932 (0.769 - 1.130) | 0.932 |
a Model 1 unadjusted model; model 2 was adjusted for age and gender; model 3 was adjusted for age, gender and Bun; model 4 was adjusted for age, gender and bun, previous AMI, CVD.
5. Discussion
Cardiovascular disease, depression, and anxiety are prominent contributors to disability and mortality, affecting 260 million individuals globally (8). Recent evidence has revealed that high levels of anxiety are associated with poor prognosis in patients with MI (13). Additionally, anxiety is associated with blood inflammatory markers such as RDW (9). Given the importance of this issue, in this study, we decided to evaluate RDW as a predictive serum marker for post-AMI anxiety. Based on different analyses, our results showed no significant correlation between RDW and anxiety after AMI.
In line with our findings, Vulser et al. found no significant association between depressive and anxiety symptoms and inflammatory markers (14). In contrast, in the study by Peng et al., which investigated the relationship between RDW and post-stroke fatigue, a relation between RDW and anxiety was shown (15). It should be noted that they also used the HRSA. The study by Shafiee et al. revealed that higher anxiety scores are associated with higher hematological inflammatory markers, including RDW (16). However, they used the Beck Depression and Anxiety Inventories (BDI) to assess anxiety.
In contrast to our findings, several recent studies have hypothesized that high RDW levels among individuals with anxiety may predict the risk of CVDs (17, 18). Moreover, Fábián et al. indicated that RDW has no predictive impact on patients with anxiety based on BDI (11). In our study, most of the patients who experienced anxiety were men, whereas in other studies, anxiety following AMI seems to disproportionately impact women (8).
A previous study indicated that increased RDW may result from an underlying inflammatory state associated with negative outcomes in patients (19). Some studies have demonstrated that RDW measurements are useful in differentiating individuals with various psychiatric disorders (20, 21). A significant finding in our study was the higher BUN level in patients with post-AMI anxiety. Interestingly, consistent with our findings, Qawaqzeh et al. showed that a decreased BUN level was a predictor of increased depressive and anxiety symptoms (22). Since no similar study has investigated the relationship between anxiety, RDW, and AMI, we could not accurately compare the results of our study with others.
5.1. Conclusions
The present study is the first to determine the predictive value of RDW on post-AMI anxiety. Based on our findings, no significant relationship was observed between RDW and anxiety following AMI. Therefore, conducting more studies in this field would be helpful in determining whether this marker should be used for anxiety prediction after AMI.