1. Background
Acute respiratory diseases are among the most common human illnesses and a significant cause of disability. Respiratory infections also account for a high proportion of hospitalizations and deaths (1). Various microorganisms can cause such infections, including coronaviruses (2). At the end of December 2019, the first outbreak of coronavirus disease 2019 (COVID-19) was reported in Wuhan, China (3). In Iran, more than 97,424 people were infected with this virus, with a mortality rate of 6.83% (4).
Most affected patients initially presented with symptoms such as fever and mild to moderate respiratory manifestations, and varying degrees of pulmonary involvement were subsequently identified on lung computed tomography (CT) scans (5). Computed tomography scans play a confirmatory role in determining the severity of the disease (6). The most common patterns on CT scans include diffuse, typically bilateral, patchy ground-glass opacities, consolidation, and/or interstitial opacities (7-9).
Older males with underlying comorbidities, particularly diabetes, hypertension, obesity, and cardiopulmonary diseases, are at a higher risk of severe complications. Concerns about the consequences of COVID-19 in immunocompromised patients arose early in the pandemic, with conflicting data observed regarding COVID-19 complications in these patients (10, 11). Individuals with immunodeficiency are at a higher risk of superinfection with bacterial and fungal microorganisms compared to those with a healthy immune system. This increased risk is due to underlying conditions such as malignancy and other causes of immunodeficiency, including HIV, organ transplantation, and the use of immunosuppressant drugs (12, 13).
Most studies in this field have been conducted on the general population with normal immune systems, and few have examined CT scan findings in immunodeficient patients with COVID-19.
2. Objectives
Given the importance of this issue and the conflicting findings in recent studies, we evaluated lung CT scan findings in this group of patients with COVID-19 compared to immunocompetent patients.
3. Methods
3.1. Participants
In this cross-sectional study, 1,246 patients infected with COVID-19, both with healthy immune systems and immunodeficiencies, who were referred to Shahid Beheshti Hospital in Hamadan Province, Iran, between 2019 and 2022, were investigated through simple random sampling. Patients with a confirmed diagnosis of COVID-19 based on a positive real-time polymerase chain reaction (PCR) test and aged at least 18 years were included in the study. Exclusion criteria included lack of access to patients' medical records due to missing information or any other reason, and having chronic parenchymal lung diseases.
3.2. Data Collection
Demographic characteristics, clinical symptoms (dyspnea, cough, headache, and fever), and history of underlying diseases were recorded. For all patients with a confirmed diagnosis of COVID-19 (PCR positive) based on the definitions of the national guidelines, lung CT scans were performed during hospitalization and upon arrival at the hospital. The images were evaluated both quantitatively and qualitatively. Types of lung involvement were categorized as airway abnormality, ground-glass opacity (GGO), consolidation, crazy-paving pattern, mixed GGO, tree-in-bud, pure GGO, patchy consolidation, adenopathy, cavitation, pleural effusion, and subpleural reticular band.
For the quantitative evaluation of lung involvement and to compare the extent of involvement, the lung was divided into three parts: Upper (above the carina), mid (between the carina and the inferior pulmonary vein), and lower (below the inferior pulmonary vein). The severity of lung involvement was graded as minimal (< 25%), mild (25 - 50%), moderate (50 - 75%), and severe (> 75%). All these evaluations were conducted by a radiologist. Finally, all the studied variables were compared between patients with immune system deficiencies and those without.
3.3. Statistical Analysis
Qualitative variables were expressed as frequency (percentage), and quantitative variables were expressed as mean ± standard deviation. ANOVA, chi-square, or exact tests were used for statistical comparisons. Additionally, logistic regression analyses were conducted to assess the associations between the study parameters and the immune system status in the two groups. All statistical analyses were performed using SPSS software version 22 and STATA software (StataCorp LLC, Texas, USA), version 15.1.
4. Results
A total of 1,246 patients participated in this study, of which 623 (50%) were women. The mean age of the participants was 51 ± 15.4 years, and the mean duration of hospitalization was 8.8 ± 5.8 days. Table 1 shows that the prevalence of a history of diabetes, hypertension (HTN), malignancy, and kidney dysfunction was higher in patients with immune system deficiency (P < 0.05) and in patients over 50 years old. Among clinical manifestations, dyspnea, cough, and fever were more frequent; however, there was no significant difference among them (P > 0.05).
| Variables | Immune System Deficiency | P-Value | |
|---|---|---|---|
| Yes | No | ||
| Gender | 0.37 | ||
| Male | 298 (47.8) | 325 (52.2) | |
| Female | 379 (60.9) | 244 (39.1) | |
| Age | 0.002 b | ||
| < 50 years | 246 (40.3) | 460 (59.7) | |
| > 50 years | 433 (56.6) | 107 (43.4) | |
| CVDs | 27 (100) | 0 (0) | 1 |
| DM | 162 (100) | 0 (0) | 0.02 b |
| HTN | 216 (100) | 0 (0) | 0.005 b |
| Malignancy | 325 (100) | 0 (0) | 0.001 b |
| Past medical history | |||
| Autoimmune diseases | 27 (100) | 0 (0) | 1 |
| Infectious diseases | 27 (100) | 0 (0) | 1 |
| Kidney dysfunction | 298 (100) | 0 (0) | 0.001 b |
| Asthma | 27 (100) | 0 (0) | 1 |
| Immunosuppresor use | 433 (100) | 0 (0) | 0.001 b |
| Clinical symptoms | 0.92 | ||
| Dyspnea | 27 (50) | 27 (50) | |
| Cough | 81 (75) | 27 (25) | |
| Headache | 27 (50) | 27 (50) | |
| Fever | 27 (50) | 27 (50) | |
| Dyspnea,cough | 162 (66.7) | 81 (33.3) | |
| Dyspnea,cough ,fever | 81 (42.9) | 108 (57.1) | |
Demographic and Clinical Information of Patients a
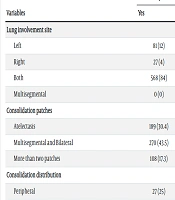
Based on chi-square test results, the groups compared showed no significant difference in terms of spiral lung CT scan findings (P > 0.05). Additionally, the severity of lung involvement was minimal in most patients. Further details are provided in Table 2.
| Variables | Immune System Deficiency | P-Value | |
|---|---|---|---|
| Yes | No | ||
| Lung involvement site | 0.30 | ||
| Left | 81 (12) | 0 (0) | |
| Right | 27 (4) | 27 (4.8) | |
| Both | 568 (84) | 541 (95.2) | |
| Multisegmental | 0 (0) | 27 (5.9) | |
| Consolidation patches | 0.97 | ||
| Atelectasis | 189 (30.4) | 162 (35.3) | |
| Multisegmental and Bilateral | 270 (43.5) | 216 (47.1) | |
| More than two patches | 108 (17.3) | 54 (11.8) | |
| Consolidation distribution | 0.99 | ||
| Peripheral | 27 (25) | 27 (37.5) | |
| Peripheral and Central | 81 (75) | 135 (62.5) | |
| GGO patches | 0.99 | ||
| Multisegmental and Bilateral | 569 (84) | 514 (90.5) | |
| Multisegmental and Unilateral | 27 (4) | 27 (4.8) | |
| GGO distribution | 0.69 | ||
| Peripheral | 433 (64) | 406 (71.4) | |
| Peripheral and Central | 243 (36) | 162 (28.6) | |
| Mixed GGO | 1.0 | ||
| Yes | 514 (76) | 433 (76.2) | |
| Crazy paving | 0.51 | ||
| Unilateral | 54 (40) | 27 (12.5) | |
| Bilateral | 81 (60) | 190 (87.5) | |
| Reverse hallo | 1.0 | ||
| Yes | 54 (8) | 54 (9.5) | |
| Pleural effusion | 0.23 | ||
| Bilateral | 27 (4) | 0 (0) | |
| Unilateral | 81 (12) | 0 (0) | |
| Emphysematous changes | 0.49 | ||
| Yes | 54 (8) | 0 (0) | |
| Lymphadenopathy | 0.49 | ||
| Mediastinal | 54 (8) | 0 (0) | |
| Location | 0.47 | ||
| Axillary | 27 (4) | 0 (0) | |
| Mid zone and Lower zone | 190 (28) | 216 (38.1) | |
| Upper zone, Mid zone and Lower zone | 406 (60) | 325 (57.1) | |
| Total lung involvement score | 0.51 | ||
| Minimal | 298 (44) | 298 (52.4) | |
| Middle | 190 (28) | 81 (14.3) | |
| Moderate | 81 (12) | 135 (23.8) | |
| Severe | 108 (16) | 54 (9.5) | |
| Atelectasis | 0.76 | ||
| Yes | 243 (36) | 243 (42.9) | |
Comparison of the Studied Groups in Terms of Spiral Lung CT Scan Findings a
Moreover, we evaluated CT scan findings between male and female patients, and the results showed no significant difference between the two genders, except for mixed ground-glass opacity (GGO) (P < 0.05), which was more common in women. A comparison between patients under and over 50 years of age using the chi-square test indicated no significant difference between these two age groups (P > 0.05).
5. Discussion
Since concerns about the consequences of COVID-19 in immunocompromised patients were raised early in the pandemic (10, 11), and because people with immunodeficiency were at a higher risk of superinfection compared to patients with a healthy immune system (12, 13), this study compared lung CT scan findings between immunocompromised and immunocompetent COVID-19 patients.
Based on our findings, there were no differences in spiral lung CT scan findings between patients with immune system deficiency and those without. Zhu et al. conducted a study in China and revealed that the severity of pneumonia in immunocompromised COVID-19 patients was higher than in patients with normal immunity, with a mortality rate of 10% in immunocompromised patients with severe pulmonary involvement (14). Conversely, Baek et al. showed that immune system deficiency was associated with a higher risk of death in patients with COVID-19 (15). The different results in these studies may be due to the use of different definitions and classifications for immunosuppression (16). It should be noted that in most studies conducted in this field, only a very small number of patients with immune system defects have participated. One systematic review showed that immunosuppression may not be related to the severity of COVID-19 but is related to the outcomes associated with other diseases in people with COVID-19 (17). The findings of Denis et al.'s study demonstrated the necessity of providing the same care for patients with immunodeficiency as for those without it during this pandemic (18).
In this study, no difference in lung CT scan findings, except for mixed GGO, was observed between male and female patients. In contrast, another study found that peripheral distribution of opacities was more common in men, and women under 60 years had lower total lung score involvement (19). It should be noted that using CT scans as the sole method for diagnosing COVID-19 may lead to errors due to similarities with the radiological findings of other lung infections and may not be able to diagnose the disease in its early stages (20).
There are a number of important limitations to consider in this study. First, due to the geographical area and time frame of the study, there was limited access to data and a smaller sample size. Second, since the information was gathered from patients' medical records, we were unable to access background variables, which would have allowed us to investigate their effect on the comparison of CT scan findings.
5.1. Conclusions
This paper aimed to compare lung CT scan findings in immunocompromised and immunocompetent COVID-19 patients. The findings suggest that these two groups do not differ in terms of spiral lung CT scan findings. Based on the results of the present study, conducting a meta-analysis in the future would be beneficial for obtaining more accurate results.