1. Background
Fungal contamination of surgical instruments is a significant concern in healthcare settings due to the risk of postoperative infections, which can result in severe complications and increased mortality among patients. Despite strict sterilization protocols, the persistence of fungal contaminants on surgical instruments poses a critical challenge, necessitating continuous monitoring and improvement of sterilization techniques. Ensuring the sterility of surgical instruments is essential for maintaining patient safety and preventing nosocomial infections (1). Fungal infections can be particularly problematic because of fungi's ability to form biofilms, which shield them from standard sterilization methods (2). Common fungal contaminants, such as Aspergillus and Candida species, are frequently identified in healthcare settings, and their presence on surgical instruments can lead to invasive infections, especially in immunocompromised patients (3). The consequences of these infections include longer hospital stays, increased healthcare costs, and higher patient mortality rates. Thus, understanding the frequency and sources of fungal contamination is crucial for developing effective strategies to mitigate this risk (4).
The prevalence of healthcare-associated infections (HAIs) varies by region and healthcare setting. According to the World Health Organization (WHO), the prevalence of HAIs in high-income countries is approximately 7%, while in low- and middle-income countries, it reaches 15% (5). The most common types of HAIs include urinary tract infections, surgical site infections, bloodstream infections, and pneumonia—especially ventilator-associated pneumonia. The prevalence of these infections can vary depending on the type of healthcare facility and the patient population (6). Studies conducted in Iran report hospital infection rates ranging from 0.32% to 9.1%. The most common infections involve the urinary tract, with Escherichia coli being the most frequently isolated microorganism. Nosocomial infections are most prevalent in special care units, internal medicine, and hematology departments, with suction devices identified as a key risk factor in Iran (7).
Fungal pathogens, while often under-recognized, are an important cause of hospital-acquired infections. These infections pose severe risks, especially for immunocompromised patients and those with prolonged hospital stays. The incidence of fungal HAIs has risen in recent years due to factors such as the growing population of immunocompromised patients, the use of broad-spectrum antibiotics, and the increased use of invasive medical procedures (8, 9). Hospital-acquired fungal infections can significantly impact patient health, often leading to severe complications and elevated mortality rates. They complicate the treatment process, particularly for patients with weakened immune systems (10). Fungal HAIs frequently result in extended hospitalizations, which prolong the time required for diagnosis and treatment, thereby delaying recovery from the primary condition. Prolonged hospital stays also heighten the risk of additional infections and impose a psychological burden on patients due to the extended duration of their treatment (9, 11).
2. Objectives
This study aimed to investigate the frequency of fungal contamination on surgical instruments in the operating rooms of hospitals affiliated with Zahedan University of Medical Sciences. By identifying the prevalence and types of fungal contaminants, this research seeks to highlight potential errors in sterilization processes and suggest improvements to current protocols.
3. Methods
3.1. Sample Collection
This study was conducted over a period of three months, from December 2023 to February 2024. Samples were collected at various times from all operating rooms of the hospital during both morning and evening shifts on a weekly basis. Over the three-month sample collection period, a total of 168 samples were taken from 7 operating rooms, including the emergency operating room, three general surgery rooms, two gynecological operating rooms, and one urology operating room, all under sterile conditions. To avoid any statistical bias and ensure balance in the sampling process, an effort was made to sample surgical instruments from each operating room in equal proportions. The samples for this research were randomly selected.
3.2. Culture and Determining of Fungal Contamination
After sample collection, the samples were transferred to Sabouraud Dextrose Agar (SDA) with chloramphenicol for culturing and determining the level of fungal contamination. In the next step, 65 g of the prepared medium (Q-Lab) was dissolved in 1 liter of distilled water and brought to a boil to ensure complete dissolution. The medium was then sterilized by autoclaving at 121°C for 15 minutes. After culturing the samples in SDA with chloramphenicol, they were incubated at 25 - 30°C for up to 4 weeks, with periodic checks for fungal growth each day. Due to the slow growth rate of some fungal species, all samples were kept for the full 4 weeks and were discarded only after no growth was confirmed. The type of yeast or filamentous fungi, colony appearance, and other characteristics of each sample were recorded.
3.3. Data Analysis
The data were analyzed using SPSS version 26. Descriptive statistics and other relevant statistical analyses were performed.
4. Results
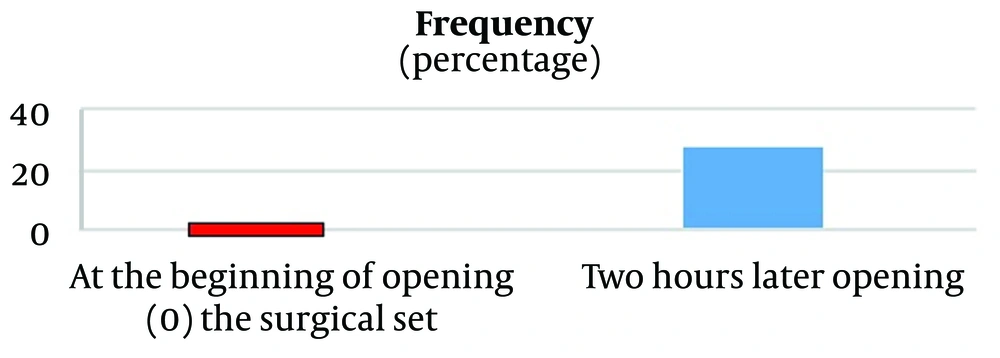
As shown in Figure 1, none of the surgical instruments had fungal contamination at the time of opening the surgical set. However, 28 surgical instruments showed fungal contamination two hours after opening the sterile sets. The contaminated instruments included Pinzette (4 instances, 14%), Allis (5 instances, 18%), Babcock Forceps (4 instances, 14%), Curved Clamp (3 instances, 11%), Gully Pot (4 instances, 14%), Receiver (1 instance, 4%), Retractor (3 instances, 11%), Ring Forceps (2 instances, 7%), and Towel Clamp (2 instances, 7%).
Furthermore, two hours after opening the sets, the contamination rates recorded across different operating rooms were as follows: General surgery with 11 instances (39%), emergency room with 8 instances (29%), urology with 6 instances (21%), and gynecology with 3 instances (11%).
The identified fungal species on the surgical instruments were Aspergillus spp. (9 instances, 32%), Yeast (7 instances, 25%), Penicillium spp. (5 instances, 18%), Aspergillus spp. and Penicillium spp. (3 instances, 11%), Aspergillus spp. and Yeast (3 instances, 11%), and a combination of Aspergillus spp., Yeast, and Penicillium spp. (1 instance, 3%).
5. Discussion
Surgical instruments are essential for delivering safe and effective medical care, yet they are vulnerable to microbial contamination, posing serious health risks to patients (12). Among various microbial contaminants, fungal contamination of surgical instruments has become a significant concern in healthcare settings. Fungi thrive in moist, warm environments commonly found in surgical suites, leading to a range of infections. Despite increasing awareness of the need to clean and disinfect surgical instruments, fungal contamination remains a persistent challenge in healthcare facilities worldwide (13).
The present study was conducted to investigate fungal contamination of surgical instruments at Ali ebn Abi Talib Zahedan Hospital in 2023. The findings revealed that while none of the surgical instruments showed fungal contamination when the sterile sets were initially opened, 28 instruments became contaminated after two hours, shedding light on the dynamics of fungal contamination within operating room environments. These results underscore the importance of maintaining sterility, not only during sterilization but also throughout the handling and use of instruments in surgical settings.
Research by Lutz et al. demonstrated that fungal spores, such as those from Aspergillus species, can persist in operating room environments, potentially contaminating sterile surfaces and equipment (14). Additionally, Eggimann and Pittet highlighted the plasticity of fungal spores and their ability to form biofilms, further complicating contamination control (15). The presence of fungal contamination two hours after sterilization suggests that the operating room environment plays a crucial role in the recontamination of sterile instruments. Contributing factors may include air movement systems, the frequency of door openings, and personnel movement within the operating room (16).
The research results indicated that Allis tools (18%), Pinzette (without) (14%), and Babcock Forceps were among the instruments with the highest levels of fungal contamination two hours after opening the sterile sets. The primary reason for the contamination of these instruments is likely their prolonged exposure to the operating room environment after the sterile packages were opened. As these tools remained unused, they were increasingly exposed to environmental contamination. This aligns with the understanding that airborne fungal spores can settle on surfaces and instruments over time (17, 18). Other studies have examined the impact of air quality on fungal contamination, showing that suspended particles are a significant source of airborne pollution. The findings of this study support the idea that airborne fungal spores contribute to contamination, particularly when tools are left unused (19, 20).
A study by Stauning et al. found that operating rooms with high traffic and frequent procedural turnover had higher rates of microbial contamination (21). Similarly, the present study suggests that the gynecology, urology, and general surgery rooms had the highest number of infections.
Aspergillus spp. was the most commonly detected fungus, contaminating tools in 32% of cases. Aspergillus species are well-known for their widespread presence in the environment, particularly in dust and air (22). Their spores can survive under various conditions and are often found in hospitals (23). This high rate of contamination is concerning because Aspergillus spp. can cause invasive infections, especially in immunocompromised patients, leading to severe complications (24).
A study conducted by Fang et al. found that Aspergillus spp. and Penicillium spp. are the most common airborne fungi in enclosed environments, which aligns with the findings of this study. Their research demonstrated that these fungi are prevalent in the air and can settle on surfaces, particularly in poorly ventilated areas (25).
5.1. Conclusions
This study underscores an important aspect of surgical instrument sterilization that extends beyond the initial sterilization process. The absence of fungal contamination immediately after opening the sterile sets confirms the effectiveness of current sterilization methods. However, the recontamination observed two hours later highlights the need for more comprehensive infection control measures that address environmental factors in the operating room. By implementing advanced environmental controls, enhancing sterilization techniques, and conducting regular monitoring, healthcare facilities can better protect patients from the risks of fungal contamination and improve overall surgical outcomes.