1. Background
The hepatitis B virus (HBV) infection poses a significant health risk for healthcare workers (HCWs) due to occupational exposure to blood and body fluids (1, 2). Blood-borne HBV infections affect approximately 5.9% of HCWs annually, amounting to 66,000 infections worldwide (2). Infected HCWs may also transmit HBV to patients during surgery or exposure-prone procedures (3). For example, over eight years, a surgeon infected with HBV transmitted the virus to more than 100 individuals (4). Vaccination can prevent HBV transmission from patients to HCWs. However, not all HCWs are vaccinated or respond to vaccination as expected (4). Furthermore, even after successful vaccination, antibody levels may decrease over time (5).
Despite the high efficacy of the hepatitis B vaccine, uncertainties remain regarding the duration of its protection and the potential need for booster doses (6). Several studies have investigated the long-term effects of HBV vaccination, but the findings are inconsistent. Some studies affirm the long-term immunogenicity of the hepatitis B vaccine and conclude that booster doses are unnecessary (7-9). Conversely, other studies report that antibody levels decline over time following vaccination, suggesting a booster dose may be required (10, 11). For instance, a survey of 243 participants revealed that 90% retained evidence of protection 30 years after hepatitis B vaccination (9). However, another study found that after 15 years, only one-third of the population maintained protective antibody levels (10).
2. Objectives
In addition to these controversies, there is a paucity of research on the HBV immune status of health sciences students and the efficacy of booster doses in Iran. This study aimed to evaluate the immunization status and Hepatitis B surface antibody (anti-HBs) titers of a group of health sciences students. Furthermore, it investigated the effect of a booster dose on participants with initially low antibody levels.
3. Methods
3.1. Study Design and Population
Based on a prior study (12), the power and sample size were calculated using the appropriate sample size formula (13) with α = 0.05 and β = 0.2. This interventional study recruited participants from Aja University of Medical Sciences between January 2023 and January 2024. Participants included students from various disciplines, such as medicine, nursing, paramedicine, and dentistry. Paramedical students specialized in fields including anesthesiology, radiology, health information technology, laboratory medicine, and surgical technology. Baseline characteristics and information were collected for all participants, followed by the determination of their anti-HBs antibody levels.
Participants with insufficient antibody levels [< 10 milli-international units per milliliter (mIU/mL)] received a single dose of the hepatitis B vaccine. One month after the booster dose, the anti-HBs antibody levels were reassessed. No specific exclusion criteria were applied, ensuring a diverse representation of students across different disciplines and entry years. The procedures and purpose of the study were thoroughly explained to all participants before the study commenced, and informed consent was obtained prior to enrollment.
This study adhered to the principles of the Declaration of Helsinki. Ethical approval was granted by the Research Ethics Committee of Aja University of Medical Sciences (Approval number: IR.AJAUMS.REC.1401.156).
3.2. Data Collection
A structured questionnaire was used to collect data, including age, gender, field of study, entry year, history of any disease, and current medication use. Information about HBV vaccination in infancy, the number of HBV vaccine doses received in infancy, adherence to the correct immunization schedule, and a history of needlestick injuries was also gathered. Participants could respond to these items with "Yes," "No," or "I don't know." The correct immunization schedule was defined as three doses administered over a period of 0, 1, and 6 months, based on the recommendations of the Centers for Disease Control and Prevention (CDC) (14).
A team of trained examiners measured the participants' anthropometric characteristics, including height and weight. Weight was measured using a Calibrated Balance Beam Scale, with values rounded to the nearest 0.1 kg, while a portable stadiometer was used to measure standing height, rounded to the nearest 0.1 cm. Body Mass Index (BMI) was calculated by dividing weight (kg) by height squared (m²). Body Mass Index was categorized into three groups: Underweight (BMI < 18.5 kg/m²), normal weight (18.5 kg/m² ≤ BMI < 25 kg/m²), and overweight/obese (BMI ≥ 25 kg/m²) (15).
3.3. Laboratory Evaluation
A blood sample (approximately 5 mL) was collected from each participant. Hepatitis B surface antigen (HBsAg) was detected in the serum samples using an enzyme-linked immunosorbent assay (ELISA). The assay utilized antibodies targeting HBsAg to bind specifically to the antigen, and positive results indicated the presence of the viral antigen in the sample. Hepatitis B surface antibodies were also measured using ELISA (Pishtazteb, Tehran, Iran). Serum samples were added to microtiter plates pre-coated with HBsAg. Following incubation and washing steps, an enzyme-linked anti-human IgG antibody was added. The subsequent color change was measured spectrophotometrically to determine the concentration of anti-HBs antibodies in the samples. Strict quality control measures were implemented throughout the process to ensure the reliability and accuracy of the results.
3.4. Vaccination
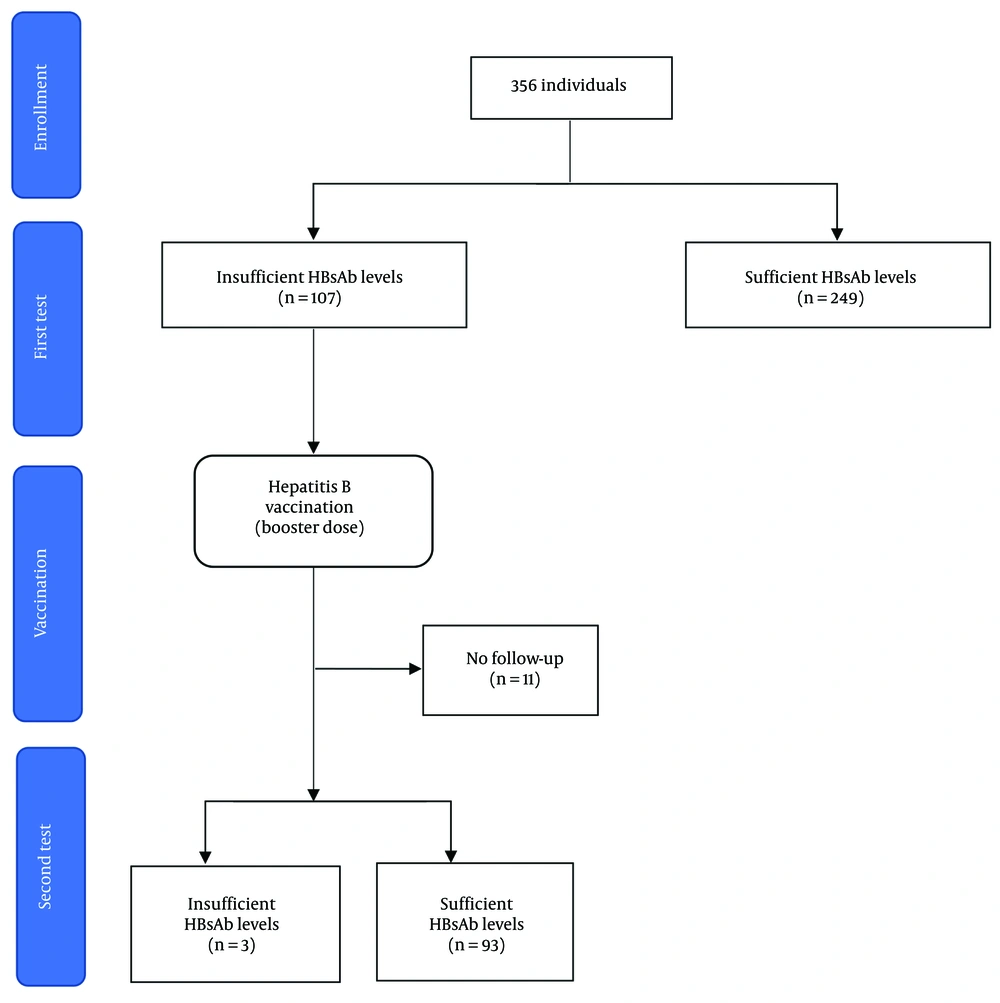
Participants with anti-HBs antibody levels < 10 mIU/mL received a booster dose of the recombinant hepatitis B vaccine (Pasteur Institute of Iran, Tehran, Iran). A 1 mL dose of the vaccine, containing 20 μg of recombinant HBsAg, was administered intramuscularly (IM) into the deltoid muscle of each participant. The research team advised participants to promptly report any unexpected symptoms or refer to a healthcare center following vaccination. Hepatitis B surface antibody levels were re-evaluated four weeks post-vaccination using ELISA, as previously described. A flow diagram outlining participant enrollment, intervention, and follow-up is provided in Figure 1.
3.5. Statistical Analysis
Data were analyzed using SPSS version 24 (IBM Corporation, New York, USA), and figures were created using GraphPad Prism version 9.4 (GraphPad Software, San Diego, CA, USA). The Kolmogorov-Smirnov test was employed to assess the normality of data distribution. Continuous variables were presented as means (± standard deviation), while categorical variables were expressed as frequencies and proportions. The chi-square test was used to compare categorical variables, and the t-test was applied to compare the means between groups.
Initially, participants were divided into two groups based on their anti-HBs levels: Insufficient anti-HBs (< 10 mIU/mL) and sufficient anti-HBs (≥ 10 mIU/mL). These groups were compared for baseline characteristics. Subsequently, the group with sufficient anti-HBs levels was further classified into two subgroups (10 mIU/mL < anti-HBs < 100 mIU/mL and 100 mIU/mL ≤ anti-HBs), and comparisons were made between these subgroups. The same analysis was repeated for participants following vaccination. Statistical significance was set at a P-value < 0.05.
4. Results
4.1. Baseline Characteristics
A total of 356 individuals participated in this study, comprising 289 males (81.2%) and 67 females (18.8%). All participants tested negative for HBsAg. None of the participants had previously checked their anti-HBs levels before this study. Approximately 97.2% of the participants reported receiving the HBV vaccine during infancy. Additionally, 7.3% (26 individuals) reported experiencing needle stick injuries in the past. Table 1 summarizes the baseline characteristics of the participants, presented both overall and stratified by their immunity status.
| Variables | Total (N = 356) | Insufficient Anti-HBs Levels (N = 107) b | Sufficient Anti-HBs Levels; (N = 249) c |
|---|---|---|---|
| Age (y) | 21.5 ± 1.8 | 21.2 ± 1.6 | 21.6 ± 1.8 |
| Gender | |||
| Male | 289 (81.2) | 86 (80.4) | 203 (81.5) |
| Female | 67 (18.8) | 21 (19.6) | 46 (18.5) |
| Field of study | |||
| Medicine | 164 (46.1) | 50 (46.7) | 114 (45.8) |
| Nursing | 112 (31.5) | 32 (29.9) | 80 (32.1) |
| Para medicine | 65 (18.3) | 20 (18.7) | 45 (18.1) |
| Dentistry | 15 (4.2) | 5 (4.7) | 10 (4.0) |
| Year of study | |||
| First | 133 (37.4) | 42 (39.2) | 91 (36.5) |
| Second | 103 (28.9) | 27 (25.2) | 76 (30.5) |
| Third | 69 (19.4) | 21 (19.6) | 48 (19.3) |
| Fourth and above | 51 (14.3) | 17 (15.9) | 34 (13.6) |
| Weight (kg) | 73.1 ± 13.3 | 73.1 ± 14.0 | 73.0 ± 13.0 |
| Height (cm) | 176.2 ± 9.4 | 176.0 ± 9.8 | 176.3 ± 9.2 |
| BMI (kg/m2) | 23.4 ± 3.3 | 23.5 ± 3.3 | 23.4 ± 3.3 |
| BMI category | |||
| Underweight | 16 (4.5) | 6 (5.6) | 10 (4.0) |
| Normal | 241 (67.7) | 71 (66.4) | 170 (68.3) |
| Overweight and obese | 99 (27.8) | 30 (28.0) | 69 (27.7) |
| Infancy vaccination | |||
| Yes | 346 (97.2) | 106 (99.1) | 240 (96.4) |
| No | 0 (0) | 0 (0) | 0 (0) |
| Don’t Know | 10 (2.8) | 1 (0.9) | 9 (3.6) |
| Number of doses in infancy | |||
| One | 8 (2.2) | 2 (1.9) | 6 (2.4) |
| Two | 37 (10.4) | 9 (8.4) | 28 (11.2) |
| Three | 243 (68.3) | 77 (72.0) | 166 (66.7) |
| Don’t know | 68 (19.1) | 19 (17.8) | 49 (19.7) |
| Correct immunization schedule | |||
| Yes | 214 (60.1) | 70 (65.4) | 144 (57.8) |
| No | 33 (9.3) | 5 (4.7) | 28 (11.2) |
| Don’t know | 109 (30.6) | 32 (29.9) | 77 (30.9) |
| History of needle stick injuries | |||
| Yes | 26 (7.3) | 5 (4.7) | 21 (8.4) |
| No | 330 (92.7) | 102 (95.3) | 228 (91.6) |
Abbreviations: Anti-HBs, hepatitis B surface antibody; BMI, Body Mass Index.
a Values are expressed as mean ± standard deviation or number (%).
b < 10 mIU/mL.
c ≥ 10 mIU/mL.
Upon assessing anti-HBs antibody levels, 249 participants (69.9%) demonstrated sufficient antibody levels (≥ 10 mIU/mL), while 107 participants (30.1%) exhibited insufficient levels (< 10 mIU/mL). No significant differences were observed between these two groups regarding the study variables (Figure 2).
Among those with sufficient antibody levels (≥ 10 mIU/mL), participants were further categorized into two subgroups based on their anti-HBs levels. The first subgroup included 187 participants (75.1%) with 10 mIU/mL < anti-HBs < 100 mIU/mL, while the second subgroup comprised 62 individuals (24.9%) with anti-HBs levels ≥ 100 mIU/mL. Comparative analysis between these subgroups revealed no significant differences in their characteristics (Table 2).
| Variables | 10 mIU/mL < Anti-HBs < 100 mIU/mL (N = 187) | 100 mIU/mL ≤ Anti-HBs (N = 62) | P-Value |
|---|---|---|---|
| Age (y) | 21.6 ± 1.8 | 21.4 ± 1.8 | 0.516 |
| Gender | 0.192 | ||
| Male | 149 (79.7) | 54 (87.1) | |
| Female | 38 (20.3) | 8 (12.9) | |
| Field of study | 0.319 | ||
| Medicine | 87 (46.5) | 27 (43.5) | |
| Nursing | 60 (32.1) | 20 (32.3) | |
| Para medicine | 34 (18.2) | 11 (17.7) | |
| Dentistry | 6 (3.2) | 4 (6.4) | |
| Year of study | 0.463 | ||
| First | 65 (34.8) | 26 (41.9) | |
| Second | 56 (29.9) | 20 (32.3) | |
| Third | 40 (21.4) | 8 (12.9) | |
| Fourth and above | 26 (13.9) | 8 (12.9) | |
| Weight (kg) | 72.7 ± 12.7 | 74.1 ± 14.0 | 0.469 |
| Height (cm) | 175.6 ± 9.2 | 178.3 ± 8.8 | 0.053 |
| BMI (kg/m2) | 23.5 ± 3.1 | 23.3 ± 4.0 | 0.707 |
| BMI category | 0.486 | ||
| Underweight | 6 (3.2) | 4 (6.5) | |
| Normal | 130 (69.5) | 40 (64.5) | |
| Overweight and obese | 51 (27.3) | 18 (29.0) | |
| Infancy vaccination | 0.213 | ||
| Yes | 178 (95.2) | 62 (100) | |
| No | 0 (0) | 0 (0) | |
| Don’t know | 9 (4.8) | 0 (0) | |
| Number of doses in infancy | 0.332 | ||
| One | 3 (1.6) | 3 (4.8) | |
| Two | 22 (11.8) | 6 (9.7) | |
| Three | 128 (68.4) | 38 (61.3) | |
| Don’t know | 34 (18.2) | 15 (24.2) | |
| Correct immunization schedule | 0.169 | ||
| Yes | 110 (58.8) | 34 (54.8) | |
| No | 17 (9.1) | 11 (17.7) | |
| Don’t know | 60 (32.1) | 17 (27.4) | |
| History of needle stick injuries | 0.901 | ||
| Yes | 16 (8.6) | 5 (8.1) | |
| No | 171 (91.4) | 57 (91.9) |
Abbreviations: Anti-HBs, hepatitis B surface antibody; BMI, Body Mass Index.
a Values are expressed as mean ± standard deviation or number (%).
4.2. After Vaccination
Participants with insufficient antibody levels (< 10 mIU/mL) received a booster dose of the hepatitis B vaccine. Of the 107 individuals, 11 participants did not return for a follow-up anti-HBs test. Among the remaining 96 participants, 93 (96.9%) achieved sufficient antibody levels after vaccination (≥ 10 mIU/mL), while three individuals (3.1%) continued to exhibit insufficient levels (< 10 mIU/mL). Analysis revealed no significant differences in demographic or baseline characteristics between these two groups.
The group with sufficient anti-HBs levels after vaccination was further divided into two subgroups: Twenty participants (24.9%) with 10 mIU/mL < anti-HBs < 100 mIU/mL and 73 participants (75.1%) with anti-HBs ≥ 100 mIU/mL. No significant differences were observed between these two subgroups regarding the study variables.
5. Discussion
This study assessed anti-HBs levels among health sciences students to evaluate HBV immunity and the effect of a booster dose on individuals with insufficient antibody levels. Among the 356 participants, 69.9% (249 individuals) demonstrated sufficient antibody levels (≥ 10 mIU/mL), with 75.1% in the range of 10 mIU/mL < anti-HBs < 100 mIU/mL and 24.9% having anti-HBs ≥ 100 mIU/mL. Meanwhile, 30.1% (107 individuals) exhibited inadequate antibody levels (< 10 mIU/mL). Participants with insufficient antibody levels received a hepatitis B vaccine booster. Of the 107 individuals, 96 underwent follow-up testing, where 93 (96.9%) achieved sufficient levels (≥ 10 mIU/mL), while three (3.1%) remained with inadequate levels (< 10 mIU/mL).
Nearly all participants in this study reported receiving HBV vaccination during infancy, consistent with previous studies in Iran (16). This high vaccination rate is largely due to a nationwide public health initiative launched in 1993, which provides vaccination to nearly all neonates (17). Enrollment in schools in Iran requires proof of vaccination. Therefore, even participants uncertain of their vaccination status were likely vaccinated.
However, hepatitis B vaccination rates vary considerably across different regions. For instance, in Cameroon, only about 11% of HCWs were fully vaccinated against HBV (18), while in Brazil, 48.9% of medical students had received the hepatitis B vaccine (19). Similarly, in the Kurdistan Region of Iraq, half of the medical sciences students were unvaccinated (20). In contrast, Europe has a higher prevalence of complete HBV vaccination among HCWs, ranging from 85% to 100% (16). These discrepancies highlight the varying levels of vulnerability among health sciences students and HCWs globally, emphasizing the critical need for enhanced vaccination initiatives in under-vaccinated regions.
In the current study, 69.9% of participants had adequate anti-HBs titers. Prior studies on health sciences students and HCWs reported varying prevalence rates of sufficient anti-HBs levels. Similar to our findings, Batra et al. observed that among vaccinated HCWs in India, 30% had anti-HBs < 10 mIU/mL, while 70% had sufficient anti-HBs levels (10.8% between 10 - 100 mIU/mL and 59.2% > 100 mIU/mL) (2). Hiva et al. reported that 83% of HCWs in Iran were serologically immune to HBV infection (16). Sukriti et al. found that 61.7% of HCWs in an Indian tertiary care hospital had protective (> 10 IU/mL) anti-HBs levels (21).
Nevertheless, some studies reported lower prevalence rates of HBV immunity among HCWs and health sciences students. For example, Mirambo et al., in a cross-sectional study in Tanzania, found that only 22% of health professional students had sufficient anti-HBs (≥ 10 IU/L). Among these, 69.4% had levels between 10 and 100 IU/L, and 30.6% had levels higher than 100 IU/L. However, these students had not received the hepatitis B vaccine during infancy (12). Similarly, Phattraprayoon et al. investigated long-term immunity among medical students and HCWs in Thailand vaccinated against HBV during infancy and reported a 49% prevalence of protective immunity (anti-HBs ≥10 mIU/mL) (5). These differences likely reflect variations in national vaccination programs and protocols across regions. Additionally, anti-HBs levels naturally decline over time after vaccination (22, 23), meaning differences in participants' ages and the interval between vaccination and antibody assessment may explain some of the observed disparities.
Despite these differences, a consistent finding across studies is that a considerable proportion of health sciences students or HCWs demonstrate insufficient anti-HBs levels. Immunity acquired through vaccination during infancy may not provide lifelong protection against HBV (5). Previous research shows that anti-HBs levels decline with time following vaccination (22, 23). Studies have demonstrated that individuals vaccinated more than ten years ago tend to have lower anti-HBs titers compared to those vaccinated within the past five years (21). For instance, a study of medical students and HCWs found that 20% and 27% lacked protection 5 and 10 years after vaccination, respectively (22). Current evidence underscores the importance of conducting antibody tests post-vaccination, particularly for HCWs, to confirm established immunity against HBV (16).
The decision to administer a booster hepatitis B vaccine to individuals with insufficient anti-HBs levels despite completing the full vaccination regimen remains a complex issue. There is ongoing debate regarding the benefits of a booster dose for HCWs with inadequate antibody levels. While some researchers argue against the need for booster doses, others advocate for them, particularly for individuals at ongoing risk of exposure (5, 7-11, 24, 25). Some studies even recommend mandatory booster doses for healthcare professionals (22). However, many studies supporting booster doses lack prolonged follow-up periods for participants.
In this study, participants with insufficient anti-HBs levels received a booster dose, resulting in 96.9% achieving adequate antibody levels. These findings align with those of Costa et al., who reported a 95% response rate to a booster dose in individuals with inadequate antibody levels (25). Similarly, Hiva et al. documented that 91% of participants with initially insufficient anti-HBs levels reached sufficient levels following a booster dose (16). Bruce et al. observed an 88% response rate to a booster dose among participants with anti-HBs levels below 10 mIU/mL (9).
Prior studies have demonstrated that declining anti-HBs levels may increase infection risk, as reduced neutralizing antibodies might be insufficient to prevent HBV infection (26). Consequently, a booster dose could benefit individuals at high risk who exhibit inadequate antibody levels. However, other studies suggest that initial vaccination provides adequate protection even in the absence of detectable anti-HBs levels, with evidence of persistent immunological memory (25, 27). Moreover, long-lasting cellular immunity conferred by vaccination may provide protection independent of anti-HBs titers (28).
Further research is necessary to clarify the necessity and efficacy of a booster dose in health sciences students and HCWs with insufficient anti-HBs titers, particularly to balance the risks and benefits in this high-risk population.
5.1. Strengths and Limitations
This study addressed an important public health concern for health sciences students, who face a heightened risk of hepatitis B infection due to occupational exposure. It took a comprehensive approach by assessing immunity status, measuring anti-HBs titers, and evaluating the impact of booster doses on participants with insufficient antibody levels. Efforts were made to ensure diversity by including students from various fields and entry years, providing a broader perspective on HBV immunity among this high-risk population.
However, the study had certain limitations. Conducting the research at a single center may limit the generalizability of the findings to HCWs or students in different regions or institutional settings. Additionally, some data were self-reported, which may introduce recall bias. The characteristics of participants who opted to participate might differ from those who declined, potentially affecting the representativeness of the sample. Furthermore, other potential risk factors contributing to low antibody levels, which were not included in the analysis, may also exist. Lastly, the short follow-up period after the booster dose may not fully capture the long-term effects and stability of antibody levels, which warrants further investigation in future studies.
5.2. Conclusions
In this study, 30% of health sciences students demonstrated inadequate (< 10 mIU/mL) anti-HBs titers. The administration of a booster dose significantly improved immunity, with 96.9% of participants achieving sufficient (≥ 10 mIU/mL) anti-HBs levels. These findings can inform the development of a protocol for HBV vaccination among students at Aja University of Medical Sciences upon their enrollment. Further research with larger sample sizes and extended follow-up periods is recommended to better assess anti-HBs titers and the long-term effects of booster doses in populations at high risk for hepatitis B.