1. Background
Food preservation has been one of humanity's greatest challenges throughout history. Turkey meat is a valuable source of muscle food due to its unique texture and high nutritional value; however, its perishability presents a significant challenge (1). In recent years, the combination of irradiation and natural plant-based coatings to extend the shelf life of food has garnered attention. Farsi gum (FG), derived from the wild almond tree, is utilized as an emulsifier in the food industry to enhance the rheological and textural properties of various products (1-5). Also known as Persian gum or Zedo gum, FG is extracted from the bark and branches of the mountain almond tree (Amygdalus scoparia), which grows naturally in the semi-arid woodlands of Iran (6, 7).
Nanoemulsions offer greater stability compared to traditional emulsions and are effective in improving the shelf life of food products (8-11). Black cumin, widely used as a spice and flavoring agent, possesses antimicrobial and antioxidant properties. Irradiation is a proven method for preserving food and is employed in over 41 countries worldwide. Given the substantial production of turkey meat in Iran, this study investigates the use of biopolymer coatings containing essential oils in conjunction with irradiation as a modern preservation method (12-14).
2. Objectives
This research evaluates the microbial, physicochemical, and sensory quality of turkey breast during chilled storage using a combination of active coating based on FG infused with black cumin essential oil (BCEO) and low-dose gamma irradiation (GIR).
3. Methods
The turkey breast meat was obtained from a local market, while the BCEO was sourced from Darin Golab Company in Kashan, Iran. The composition of the volatile oil of black cumin used in this study was analyzed using gas chromatography-mass spectrometry (GC-MS) (13). The emulsion and nanoemulsion of BCEO were prepared following the method described by Venkadesaperumal et al. (15). Particle size and zeta potential measurements were performed using a Dynamic Light Scattering (DLS) instrument (Malvern, England). The average particle diameter was determined to be 34.7 nanometers.
3.1. Preparation of Coating Solutions
The prepared emulsion or nanoemulsion was gradually incorporated into the FG solution to create a coating solution, ensuring that the final concentration of FG reached 2% and the essential oil concentration in the emulsion or nanoemulsion was 1% (based on the volatile oil content of black cumin).
3.2. Preparation of Meat Samples and Storage Conditions
Fresh turkey breast (6 hours post-slaughter) was cut into 90 to 100-gram portions using sterile utensils in the laboratory and classified into the following eight groups: (1) control (C): No treatment; (2) FG: Dipped in FG; (3) GIR: Gamma-irradiated at 2 kGy; (4) FG + BCEO: Dipped in FG containing BCEO emulsion; (5) FG + black cumin nanoemulsion (BCNE): Dipped in FG containing BCNE; (6) FG + GIR: Dipped in FG, then gamma-irradiated at 2 kGy; (7) FG + BCEO + GIR: Dipped in FG containing BCEO, then gamma-irradiated at 2 kGy; (8) FG + BCNE + GIR: Dipped in FG containing BCNE, then gamma-irradiated at 2 kGy.
The treatment groups were immersed in the respective coating solution for one minute, removed, and placed on a sieve at room temperature to allow excess solution to drain. The samples were then packed into polyethylene pouches.
The GIR procedure was performed at the Atomic Energy Organization of Iran using a Gamma Cell 220 device (Nordion Company, Ontario, Canada) with a dose rate of 4.8 Gy/s. Dosimetry was conducted using alanine dosimeters. During the irradiation process, non-irradiated samples were maintained at room temperature. Following irradiation, all samples were transported to the laboratory and stored at 3 ± 0.2°C. Microbial, physicochemical, and sensory analyses were conducted on days 0, 4, 7, 12, 15, and 21 of storage.
3.3. Enumeration of Microbial Flora
For microbial analyses, a 10 gr flesh sample was combined with 90 mL of sterile normal saline (0.85%). The mixture was homogenized using a stomacher, followed by the preparation of appropriate serial dilutions. The pour plate technique was employed to quantify total mesophilic and psychrotrophic bacteria on plate count agar following incubation at 35°C for 72 hours and at 7°C for one week, respectively. Additionally, lactic acid bacteria (LAB) were counted on MRS agar after incubation at 35°C for 72 hours. The pour-overlay technique was utilized to enumerate Enterobacteriaceae on violet red bile dextrose agar after incubating at 35°C for 24 hours.
3.4. Physicochemical Analyses
The breast samples were analyzed for pH following the method described by (16). In addition, total volatile basic nitrogen (TVB-N) was measured according to the procedure outlined in (17), thiobarbituric acid reactive substances (TBARS) were determined as described in (18), and protein carbonyls were quantified based on the methodology detailed in (19). These analyses were conducted to evaluate the physicochemical changes in the turkey breast samples during storage.
3.5. Analysis of Sensory Characteristics
A sensory analysis was performed to assess the odor, color, texture, and overall acceptability of the turkey breast samples. A panel of 20 assessors, knowledgeable about turkey meat, participated in the evaluation. A 9-Point Hedonic Scale was used, where a score of 9 indicated "like extremely" and a score of 1 represented "dislike extremely" (20). Samples with sensory scores ranging between 5 and 9 were considered acceptable in terms of sensory attributes (13).
3.6. Statistical Analysis
On each day of analysis, four independent samples from each experimental group were examined, and the resulting data were analyzed using SPSS software, version 20. Analysis of variance (ANOVA) was conducted for parametric data, including proximate composition, microbial counts, and physicochemical analyses, followed by Duncan's post hoc test to identify significant differences between groups. For nonparametric data, such as sensory analyses, the Kruskal-Wallis test was used. A P-value < 0.050 was considered statistically significant.
4. Results and Discussion
4.1. Components of Black Cumin Essential Oil
The GC-MS analysis of the essential oil derived from Iranian black cumin identified 13 phytochemical compounds (Table 1). The compounds with the highest concentrations were carvone (44.5%), γ-terpinene (20.2%), and limonene (19.9%). These findings align with previous studies (21-24).
| Compound | Retention Index | (%) Composition |
|---|---|---|
| α-Tthujene | 926 | 0.19 |
| α-Pinene | 936 | 0.95 |
| β-Sabinene | 975 | 0.54 |
| β-Pinene | 982 | 1.37 |
| β-Myrcene | 992 | 0.58 |
| ρ-Cymene | 1033 | 5.47 |
| D-Limonene | 1036 | 19.9 |
| Trans- β-Ocimene | 105 | 0.21 |
| γ-Terpinene | 1063 | 20.2 |
| Terpinene-4-ol | 1192 | 0.35 |
| Dihydrocarvone | 1213 | 1.58 |
| Trans-Dihydrocarvone | 1220 | 0.36 |
| Carvone | 1262 | 44.5 |
| Total | - | 96.2 |
4.2. Particle Size and Stability of Black Cumin Nanoemulsion
The mean particle diameter of BCNE was 34.7 nm, with a PDI of 0.437. Over 10 weeks of storage at room temperature, no signs of phase separation or creaming were observed in the BCNE.
4.3. Microbial Flora
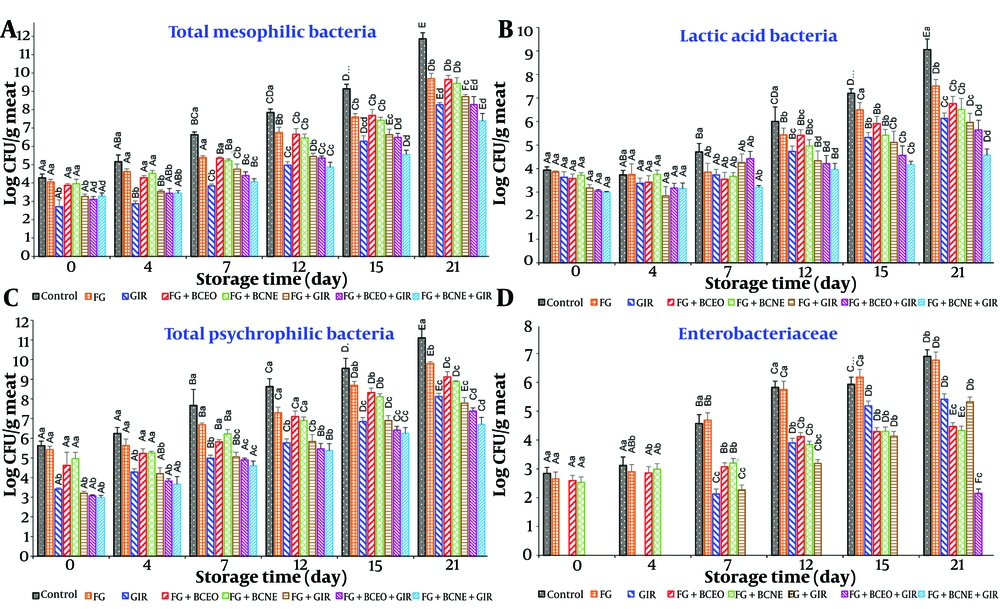
The microbial analysis of turkey breast samples across all experimental groups revealed several significant findings (Figure 1). On day 0, the mesophilic and psychrotrophic bacterial counts were significantly lower (P < 0.050) in the irradiation groups (GIR, FG + GIR, FG + BCEO + GIR, and FG + BCNE + GIR) compared to the other groups. By day 4, groups treated with free essential oil (FG + BCEO, FG + BCEO + GIR) exhibited lower bacterial counts than those treated with volatile oil nanoemulsion (FG + BCNE, FG + BCNE + GIR), likely due to the faster release of essential oil in the former. However, from day 7 onward, the nanoemulsion groups showed lower bacterial counts than the free essential oil groups, attributed to the sustained and stable release of black cumin oil from the nanoemulsion.
Effect of Farsi gum (FG) coating containing Iranian black cumin essential oil (BCEO) emulsion or black cumin nanoemulsion (BCNE) and gamma irradiation (GIR) on the microbial flora of chilled turkey breast. Different lowercase letters show significant differences among treatments at each day of the storage period, while capital letters show significant differences among the storage days in the same treatment.
Throughout the storage period, bacterial counts increased significantly (P < 0.050) across all groups. The control and FG groups exhibited higher rates of bacterial growth, demonstrating the limited effectiveness of FG alone. While there were no significant differences in bacterial counts among certain group comparisons during specific storage periods, mesophilic counts exceeded the standard limit (7 log CFU/g) in several groups by certain days. Notably, the FG + BCNE + GIR group consistently remained within acceptable limits (Figure 1).
At the end of the storage period (day 21), bacterial counts in the control and FG groups were significantly higher (P < 0.050) compared to the treated groups. Among the treatments, the FG + BCNE + GIR and FG + BCEO + GIR groups had the lowest bacterial counts, with FG + BCNE + GIR showing the most effective control over mesophilic and psychrotrophic bacteria.
Fallah et al. found that low-dose GIR, combined with a biodegradable pectin nanocomposite coating infused with curcumin nanoparticles and ajowan (*Carum copticum*) essential oil nanoemulsion, significantly reduced mesophilic and psychrotrophic bacterial levels in lamb loins during a 21-day refrigerated storage period (25). Similarly, Dini et al. reported that an edible composite film made from chitosan and cumin essential oil-loaded nanoemulsion, when paired with low-dose GIR, effectively reduced mesophilic and psychrotrophic bacterial counts in beef loins stored under refrigeration (13). Mehdizadeh et al. demonstrated that a chitosan-zein coating containing both free and nano-encapsulated extracts of Pulicaria gnaphalodes (Vent.) Boiss. effectively reduced mesophilic and psychrotrophic bacterial counts in Rainbow trout (26). Likewise, Zhang et al. showed that chitosan coatings containing either free or nano-encapsulated essential oil from Paulownia tomentosa significantly reduced mesophilic and psychrotrophic bacterial counts in ready-to-cook pork chops (27).
At the start of storage, no significant differences in LAB counts were observed among the groups, potentially due to LAB's known resistance to irradiation. Lactic acid bacteria counts increased across all groups throughout the storage period, with higher growth rates noted in the control and FG groups. This suggests that FG was not effective in inhibiting LAB proliferation. Significant differences in LAB counts were observed among the various group comparisons (Figure 1). On day 4, LAB counts were lower in groups treated with essential oil compared to those treated with volatile oil nanoemulsion, likely due to the faster release of essential oil. By day 7, LAB counts were lower in the nanoemulsion-treated groups, attributed to the stable release of essential oil from the BCNE.
At the end of the storage period (day 21), LAB counts were significantly higher in the control and FG groups compared to the treated groups, with the FG + BCNE + GIR group exhibiting the lowest LAB count (25). Although irradiated groups generally showed lower LAB counts, the differences were not statistically significant.
Regarding Enterobacteriaceae, no significant differences were observed among the control, FG, FG + BCEO, and FG + BCNE groups, with levels remaining undetectable in all irradiation groups during storage, likely due to the sensitivity of Enterobacteriaceae to GIR (Figure 1D). Counts increased significantly in the control and FG groups, while treated groups demonstrated much lower rates of bacterial growth. Overall, the combination of low-dose GIR with various coatings effectively reduced bacterial counts, highlighting its positive impact on meat preservation (28).
Gamma irradiation efficiently eliminates microorganisms by directly damaging their DNA or disrupting enzymatic systems essential for survival. Furthermore, the essential oil derived from Iranian black cumin exhibits potent antimicrobial properties, primarily by compromising the integrity of bacterial cell membranes in both Gram-positive and Gram-negative bacteria. Studies have demonstrated that nano-emulsified essential oils possess superior antimicrobial activity compared to traditional emulsions. This enhanced efficacy is attributed to the smaller particle sizes of nanoemulsions, which allow for improved penetration into bacterial cells and heightened interaction with microbial membranes (25).
4.4. Physicochemical Analyses
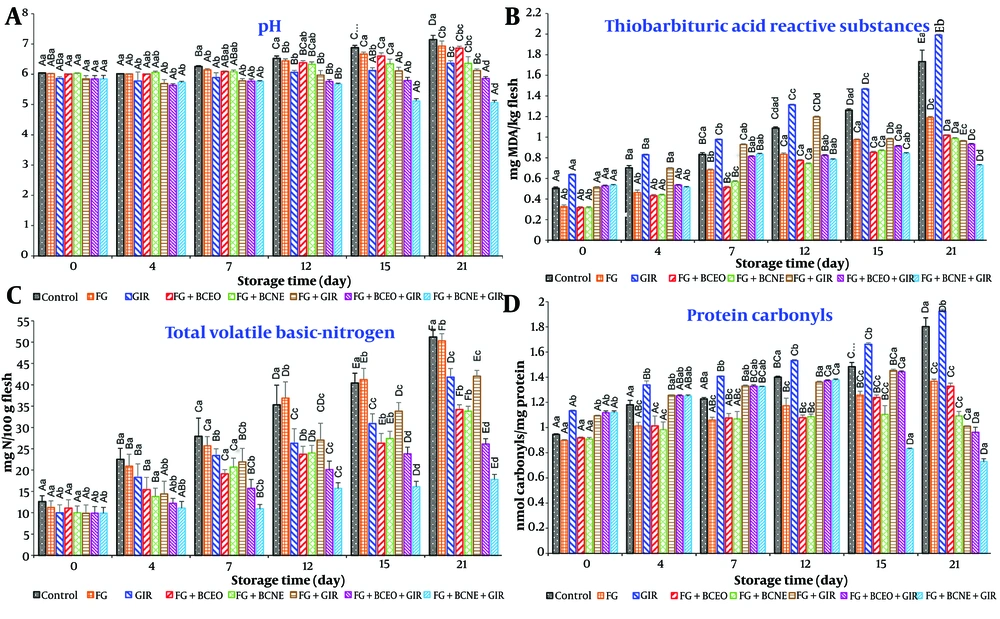
The results of the physicochemical analyses are presented in Figure 2. At the beginning of storage, no significant differences in pH levels were observed among the experimental groups (Figure 1A and B). However, by day 4, groups treated with essential oils exhibited lower pH values compared to those with nanoemulsions, which was attributed to the faster release of essential oils. From day 7 onward, nanoemulsion-treated groups showed lower pH values, suggesting a more stable and sustained release of the oil (25). By day 21, the control and untreated groups had significantly higher pH levels than the treated groups, with the FG + BCNE + GIR combination effectively controlling pH changes throughout the storage period (Figure 2) (13).
Effect of Farsi gum (FG) coating containing Iranian black cumin essential oil (BCEO) emulsion or black cumin nanoemulsion (BCNE) and gamma irradiation (GIR) on the physicochemical parameters of chilled turkey breast. Different lowercase letters show significant differences among treatments at each day of the storage period, while capital letters show significant differences among the storage days in the same treatment.
Total volatile basic nitrogen content, an indicator of meat quality, ranged from 9.81 to 12.2 mg N/100 g. Higher TVB-N levels are typically associated with increased microbial activity (Figure 2). Groups treated with free essential oils initially had lower TVB-N levels, which increased over time. However, treated groups, particularly those treated with FG + BCNE + GIR, maintained TVB-N levels within acceptable limits throughout the storage period. These results align with previous studies involving low-dose GIR and biocomposite coatings for meat storage (13, 25).
Thiobarbituric acid reactive substances, a measure of lipid oxidation, were initially higher in irradiated groups due to radiation-induced oxidation processes, with levels increasing over the storage period. However, combinations such as FG + BCNE effectively controlled lipid oxidation. The antioxidant properties of BCEO, mainly attributed to its monoterpene hydrocarbons, play a critical role in mitigating lipid oxidation (8, 11, 13, 28).
Protein carbonyl levels, an indicator of protein oxidation, were elevated in irradiated groups, likely due to oxidative degradation (Figure 2). The FG + BCNE treatment demonstrated effectiveness in reducing protein oxidation levels. This finding highlights the relationship between lipid and protein oxidation in meat, where lipid oxidation often initiates protein degradation (13, 25, 29).
Overall, these results underscore the significant impact of combining GIR with bioactive compounds like nanoemulsions in maintaining the quality and safety of meat products during chilled storage.
4.5. Sensory Analyses
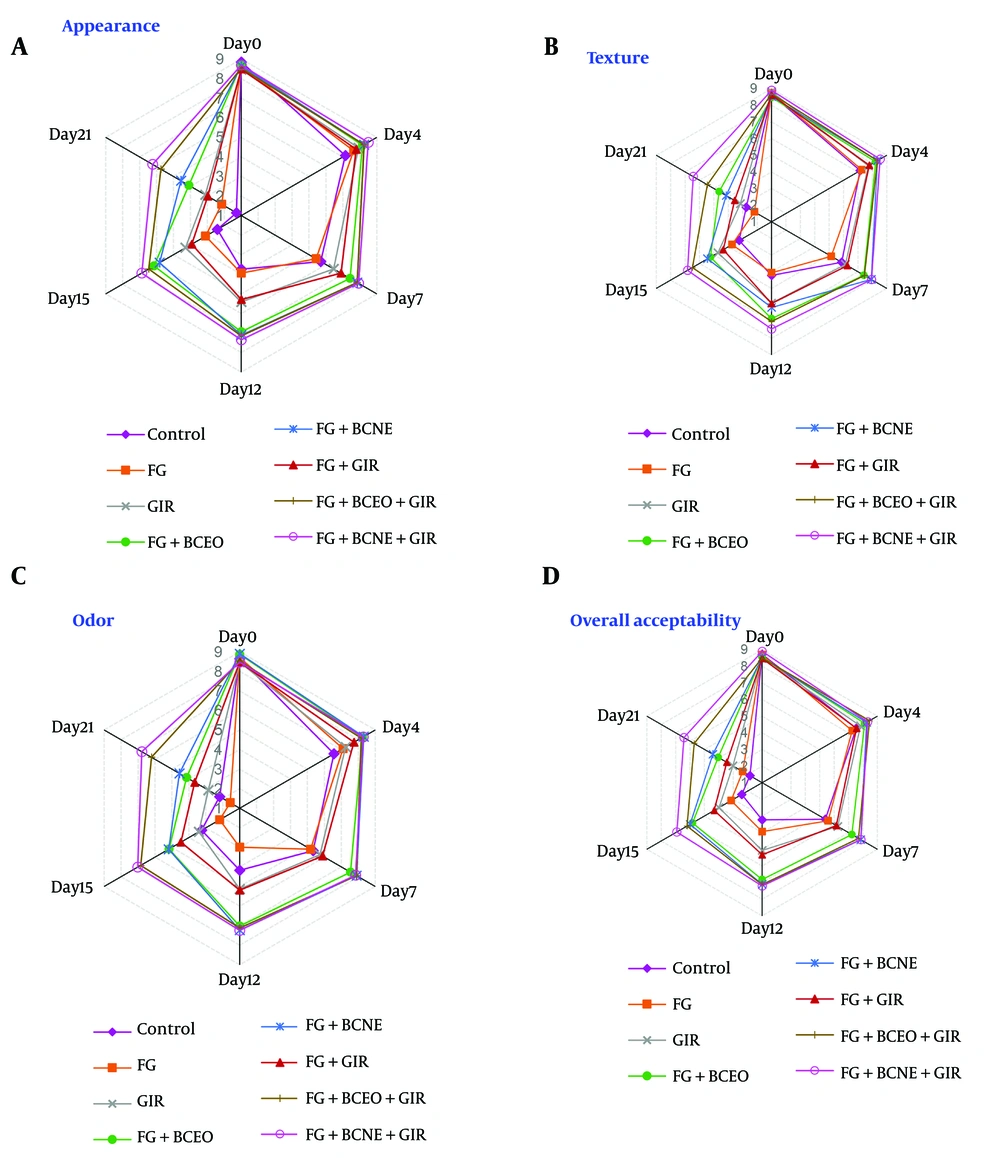
The sensory analysis of the experimental turkey breast samples evaluated attributes such as appearance, odor, texture, and overall acceptability (Figure 3). At the start of storage, all sensory attributes received high scores (greater than 8.5). However, the GIR group exhibited a slight unpleasant odor, resembling burnt or sulfur, which was likely a result of the ionizing radiation process. This odor was absent in the irradiated coated groups, suggesting that the coating effectively masked these undesirable scents.
The sensory characteristics remained acceptable (mean scores above 5) for varying durations: Seven days for the control and FG groups, 12 days for the GIR and FG + GIR groups, 15 days for the FG + BCEO and FG + BCNE groups, and 21 days for the FG + BCEO + GIR and FG + BCNE + GIR groups. Notably, no correlation was observed between the sensory analyses, particularly overall acceptability, and TMB counts or TVB-N levels. This finding suggests that even when TMB and TVB-N levels exceeded acceptable thresholds, the sensory attributes remained within an acceptable range, potentially due to the edible coating masking some sensory defects.
The ideal shelf life of turkey breast, based on parameters such as TMB, TVB-N, and overall acceptability, varied significantly among the groups: Four days for the control and FG groups, 7 days for the GIR and FG + GIR groups, 12 days for the FG + BCEO and FG + BCNE groups, 15 days for the FG + BCEO + GIR group, and at least 21 days for the FG + BCNE + GIR group.
In conclusion, this study demonstrated that the combined application of GIR at a dose of 2 kGy and an edible coating enriched with BCEO and BCNE effectively controlled microbial growth, minimized physicochemical changes, and preserved the sensory quality of turkey breast during chilled storage. The combination of an active coating containing essential oil nanoemulsion and GIR extended the chilled shelf life of turkey breast to 21 days without adversely affecting the physicochemical and sensory attributes of the samples. Consequently, the use of an active coating with FG containing the nanoemulsion of Iranian BCEO, along with low-dose GIR, is recommended for preserving turkey breast under chilled conditions.