1. Background
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia, resulting from defects in insulin secretion, insulin resistance, or both (1). Symptoms of hyperglycemia may include frequent urination, weight loss, excessive thirst, increased appetite, susceptibility to infections, ketoacidosis, and long-term complications such as retinopathy, nephropathy, and peripheral neuropathy (2). There are four types of DM. In type 1 DM, the immune system produces antibodies like Anti-GAD, ICA, and IA-2 against pancreatic β-cells, necessitating lifelong insulin therapy. Type 2 DM is characterized by insulin resistance, often seen in obese adults, which can lead to β-cell failure. Gestational DM can develop in some pregnant women due to steroid hormone production from the placenta. Special types of DM can present in a monogenic form, causing β-cell dysfunction and resulting in hyperglycemia, as seen in conditions like maturity-onset diabetes of the young (MODY) (3, 4).
In type 2 diabetes, insulin resistance occurs in muscle tissue and adipocytes. Decreased β-cell function and increased glucagon secretion are common phenomena. These patients also experience increased hepatic glucose production. Insulin binds to the β2α2 receptor in muscle, adipocytes, and liver, affecting cells via a tyrosine kinase mechanism mediated by PI3-K and PKB/Akt to facilitate glucose entry through GLUT-4 (5, 6). Insulin resistance may arise from abnormalities in insulin receptor autophosphorylation activity, elevated tyrosine phosphatase activity of PTP1β leading to insulin receptor inactivation, or phosphatase action dephosphorylating IRS-1 within the insulin signaling pathway (7).
Osteoporosis, a complication of diabetes, is characterized by decreased bone mass. Disruption in bone resorption and formation turnover leads to decreased bone density. Individuals with DM have a higher risk of developing osteoporosis, and bone tissue repair is often delayed following fractures (8, 9). During exercise, mechanical stress on the bone induces fluid flow in the lacunar-canalicular network, leading to intracellular responses such as increased intracellular calcium, matrix production, and osteogenesis (10, 11). Regular weight-bearing and muscle-strengthening exercises can help prevent osteoporosis. Walking enhances blood sugar regulation, boosts bone density through weight loss, and mitigates detrimental impacts on bones (12).
Inflammation can disrupt metabolic functions, but physical activity can increase anti-inflammatory cytokines like adiponectin, IL-4, and IL-10 while reducing pro-inflammatory cytokines such as leptin, RBP4, CRP, and TNFα. This can improve insulin sensitivity, reduce inflammation, better manage diabetes, increase bone density, control weight, and enhance bone health markers affected by inflammation (13, 14). Recently, sports medicine experts have employed innovative interventions like high-intensity interval training (HIIT) instead of continuous endurance training (CET) to manage metabolic conditions like osteoporosis and obesity. Studies suggest that HIIT may offer superior benefits in improving bone density compared to CET. This research examines how HIIT impacts osteocalcin, alkaline phosphatase, and C-telopeptide (CTX) levels, crucial factors in mitigating bone tissue degradation in diabetic individuals. In diabetic patients, markers of bone formation decrease while those of bone resorption increase, indicating a potential link between diabetes and bone fragility (15).
Bone alkaline phosphatase is a crucial marker for bone formation and is an isoenzyme distinguishable from others due to its degradation at 56°C. Osteoblasts are a significant source of alkaline phosphatase, and serum levels of this enzyme indicate osteoblastic activity (16). Osteocalcin, rich in gamma-carboxy glutamic acid, is vitamin K-dependent and a prevalent non-collagenous protein in bone, contributing to bone mineralization. Osteocalcin levels are regulated by calcitonin, parathyroid hormone, and vitamin D3 (17). It is a bone formation marker, demonstrating osteoblast activity in bone metabolism, and is also involved in glucose metabolism (17, 18).
C-telopeptide is a breakdown product from the carboxy-terminal region of collagen type I, serving as a specific serum marker for assessing bone turnover. Increased CTX levels in serum and urine indicate bone resorption (19). Irisin, a polypeptide hormone secreted from the muscle transmembrane protein FNDC5 during physical activity, increases bone formation and decreases bone resorption, promoting bone health. Irisin also facilitates the transformation of white fat into brown fat, reducing obesity, diabetes, and other health problems (20, 21).
Diabetes can impact bone health through mechanisms such as obesity, altered insulin levels, increased urinary excretion leading to reduced intestinal calcium absorption, disrupted parathyroid hormone and vitamin D homeostasis, impaired kidney function, and reduced insulin-like growth factor-i levels. In type 2 diabetes (T2DM), bone microstructure is compromised as osteoblast apoptosis increases, osteoblast differentiation decreases, and osteoclast-mediated bone resorption rises (22, 23). Exercise enhances glucose uptake into muscle cells via GLUT-4, boosts insulin receptor substrates (IRS), and promotes muscle growth. Over 75% of glucose uptake is attributed to insulin stimulation in muscle tissue, increasing tissue sensitivity to insulin (24).
Some studies suggest that HIIT has a more significant impact on metabolic function compared to CET, while others indicate no discernible difference between the two methods (25, 26). The HIIT can burn approximately 200-250 kcal in individuals (27). Bone consists of protein and minerals and contains two cell types: Osteoclasts, responsible for bone resorption, and osteoblasts, which aid in bone formation. As individuals age, osteoclast activity tends to increase, leading to decreased bone mass (28). Regular physical activity effectively manages metabolic conditions such as type 2 diabetes and obesity. Diabetic individuals are typically advised to engage in 30 minutes of low to moderate-intensity aerobic exercise (29).
2. Objectives
This study aims to investigate the impact of HIIT on the lipid profile, bone metabolism, irisin hormone, and blood sugar levels in diabetic patients. We seek to elucidate the differential effects of this exercise on diabetic patients’ health, highlighting its potential in diabetes management and bone health.
3. Methods
In this study, 55 diabetic patients with fasting blood sugar levels ranging from 126 to 250 mg/dL were examined. These patients were diagnosed with diabetes based on national guidelines and did not receive any treatment, providing personal consent. They participated in an 8-week HIIT program supervised by a sports physiologist, consisting of four sessions per week, each lasting 40 minutes and including warm-up and cool-down activities. The patients were examined by a sports physiologist following a 10 to 12-hour fasting period during which venous blood samples were collected for analysis, and anthropometric measurements were recorded. The exercise protocol consisted of a 5-minute warm-up, followed by 4 minutes of biking at 85% to 95% of maximum exercise power and then 3 minutes of relief at 60% maximum exercise power, repeated four times. This was followed by a 5-minute cool-down period (30).
The day after the final exercise session, blood samples were collected from the patients following an overnight fast for laboratory analysis. Bone alkaline phosphatase levels were measured using the inactivation method at 56°C, with enzyme activity assessed using the Pars Azmoon alkaline phosphatase measurement kit and a photometric method on the Prestige autoanalyzer. Serum levels of osteocalcin were measured using the human osteocalcin measurement kit from Siemens with a quantitative luminescence technique, while serum levels of CTX and irisin hormone were measured using the CUSABIO measurement kit employing the ELISA technique in the laboratory. All results were reported as mean ± standard deviation (SD). Group differences were assessed using the one-way ANOVA test, Tukey multiple comparison, and paired t-test in SPSS software, with significance set at P < 0.05.
4. Results
4.1. Effect of High-Intensity Interval Training on Blood Glucose Levels
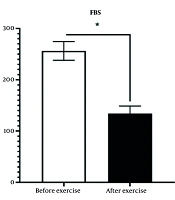
On the day preceding the commencement of training and the day following the final training session, after an overnight fast of 10 to 12 hours, 5 mL of venous blood was collected from each patient, and serum factors were analyzed using a chemical method. As shown in Figure 1, the exercise intervention significantly reduced blood sugar levels in diabetic patients. In the study group, the average fasting blood sugar was 227.8 ± 36.44 mg/dL, which decreased to 137.44 ± 17.94 mg/dL after the exercise intervention (P < 0.05) (Figure 1).
There was a significant difference in plasma glucose concentration between the diabetic group and the exercise intervention group, with a significant decrease observed in the exercise intervention group compared to the diabetic control group.
4.2. Effect of High-Intensity Interval Training on Lipid Profile
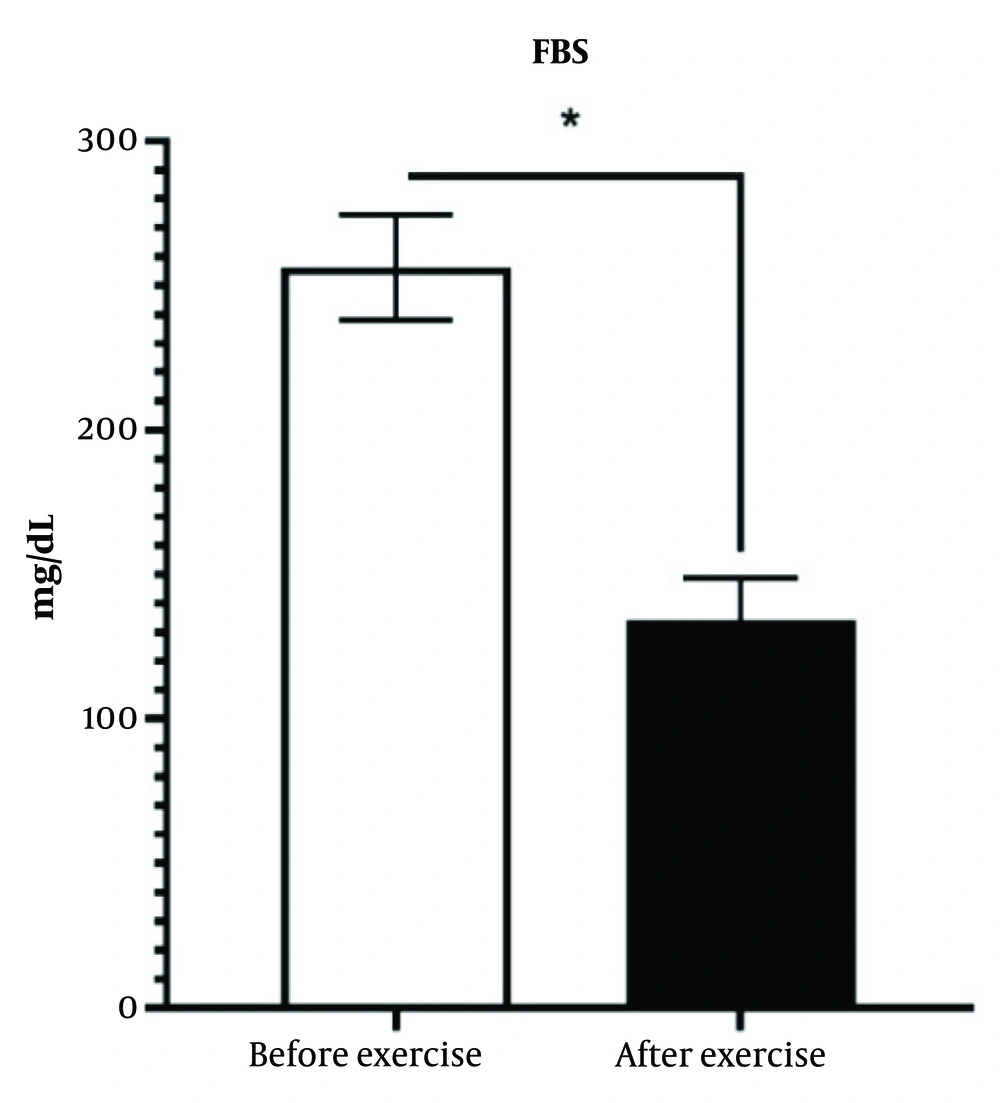
The training intervention significantly reduced the levels of total cholesterol, triglycerides, and low-density lipoprotein (LDL) cholesterol, while increasing the level of high-density lipoprotein (HDL) cholesterol in diabetic patients. In the studied group, the mean levels of total cholesterol, triglycerides, and LDL cholesterol were 212.37 ± 28.43 mg/dL, 272.11 ± 47.22 mg/dL, and 118.11 ± 30.57 mg/dL, respectively, which decreased to 175.46 ± 19.71 mg/dL, 194.26 ± 21.64 mg/dL, and 83.26 ± 30.57 mg/dL, respectively. Additionally, the HDL cholesterol level in the study group increased from 39.64 ± 5.84 mg/dL to 53.35 ± 6.57 mg/dL after the exercise intervention (P < 0.05) (Figure 2).
There was a significant difference between the diabetic group and the training intervention group, with a significant decrease in the plasma concentrations of total cholesterol, triglycerides, and LDL cholesterol observed in the training intervention group compared to the control group.
4.3. Effect of High-Intensity Interval Training on Bone Alkaline Phosphatase
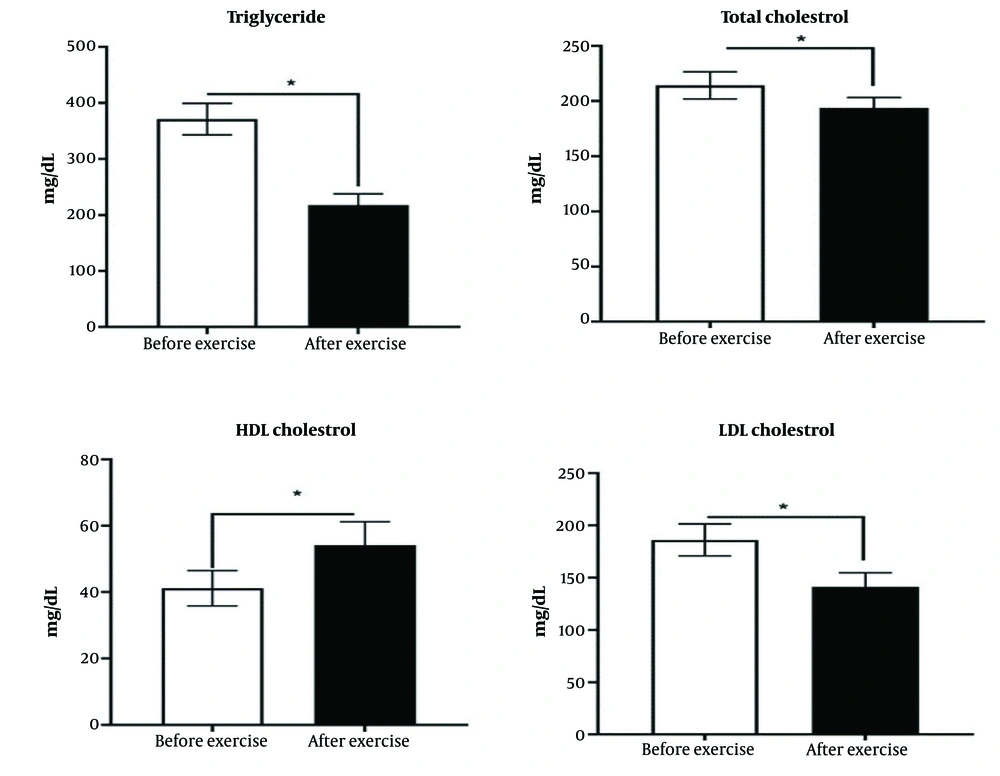
Bone alkaline phosphatase is an indicator of bone formation. The results of the study have shown that the exercise intervention has caused a significant increase in the bone alkaline phosphatase (P < 0.05) (Figure 3).
There was a significant difference between the diabetic group and the training intervention group (P < 0.05).
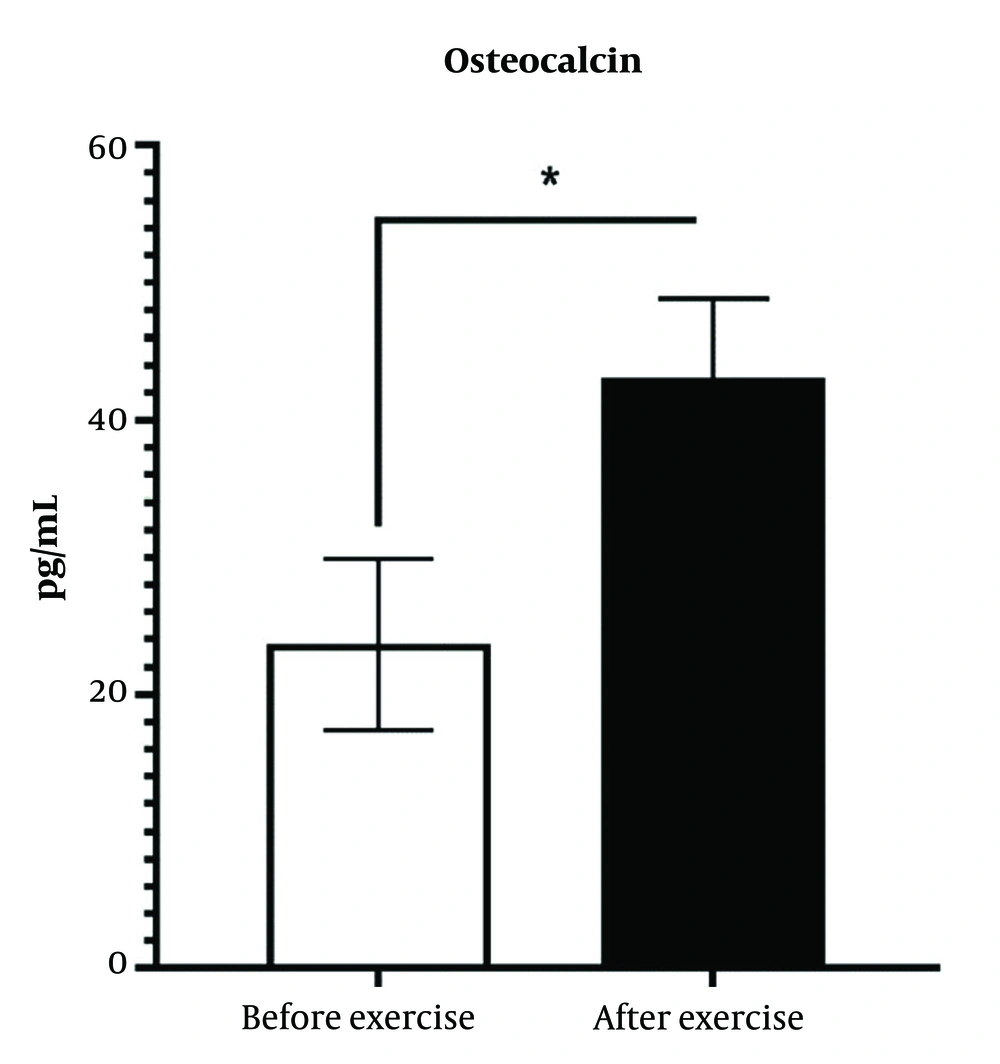
4.4. Effect of High-Intensity Interval Training on Serum Osteocalcin
High intensity interval training has significantly increased the amount of bone osteocalcin, HIIT exercise interventions have induced osteocalcin by 170% (P < 0.05) (Figure 4).
There was a significant difference between the diabetic group and the training intervention group (P < 0.05).
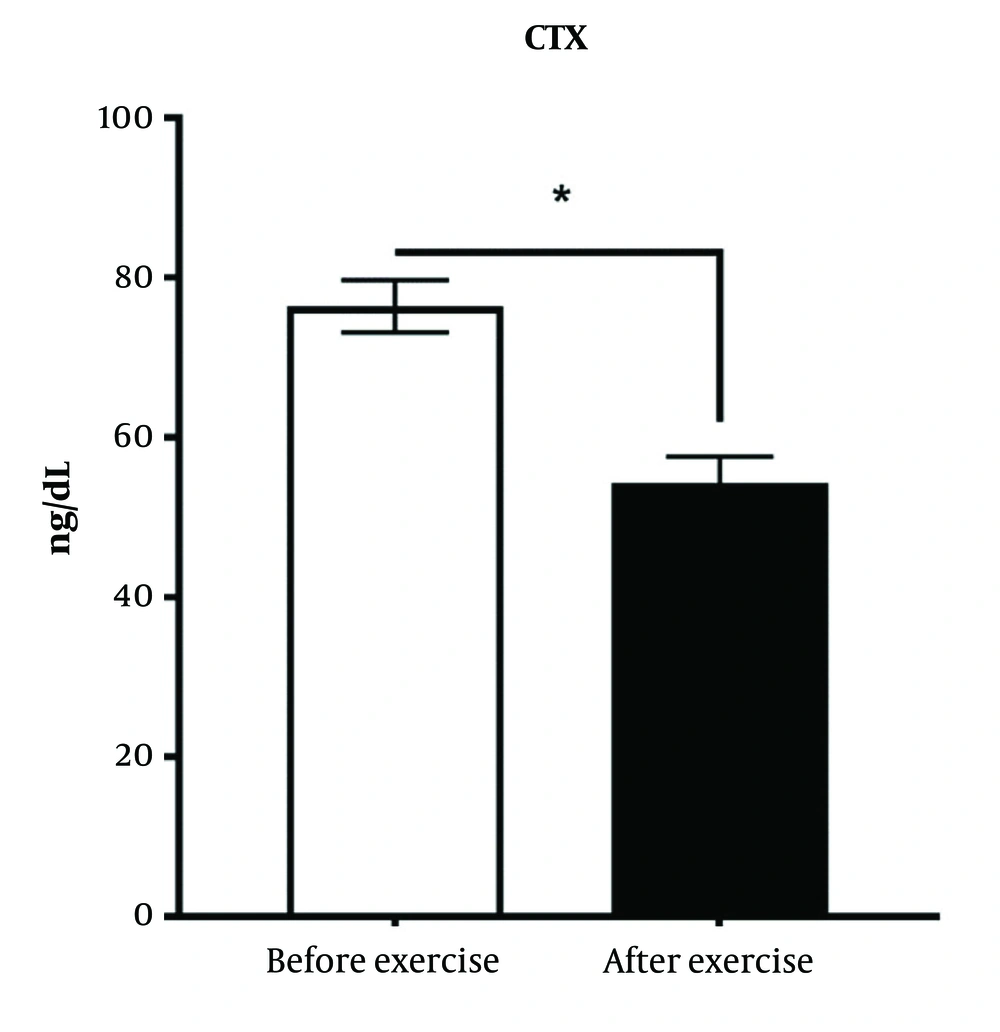
4.5. Effects of High-Intensity Interval Training Intervention on Serum C-telopeptide
C-telopeptide is one of the indicators of bone destruction. Training intervention has significantly reduced the C-Telopeptide compare to control groups (P < 0.05) (Figure 5).
There was a significant difference between the diabetic group and the exercise intervention group (P < 0.05).
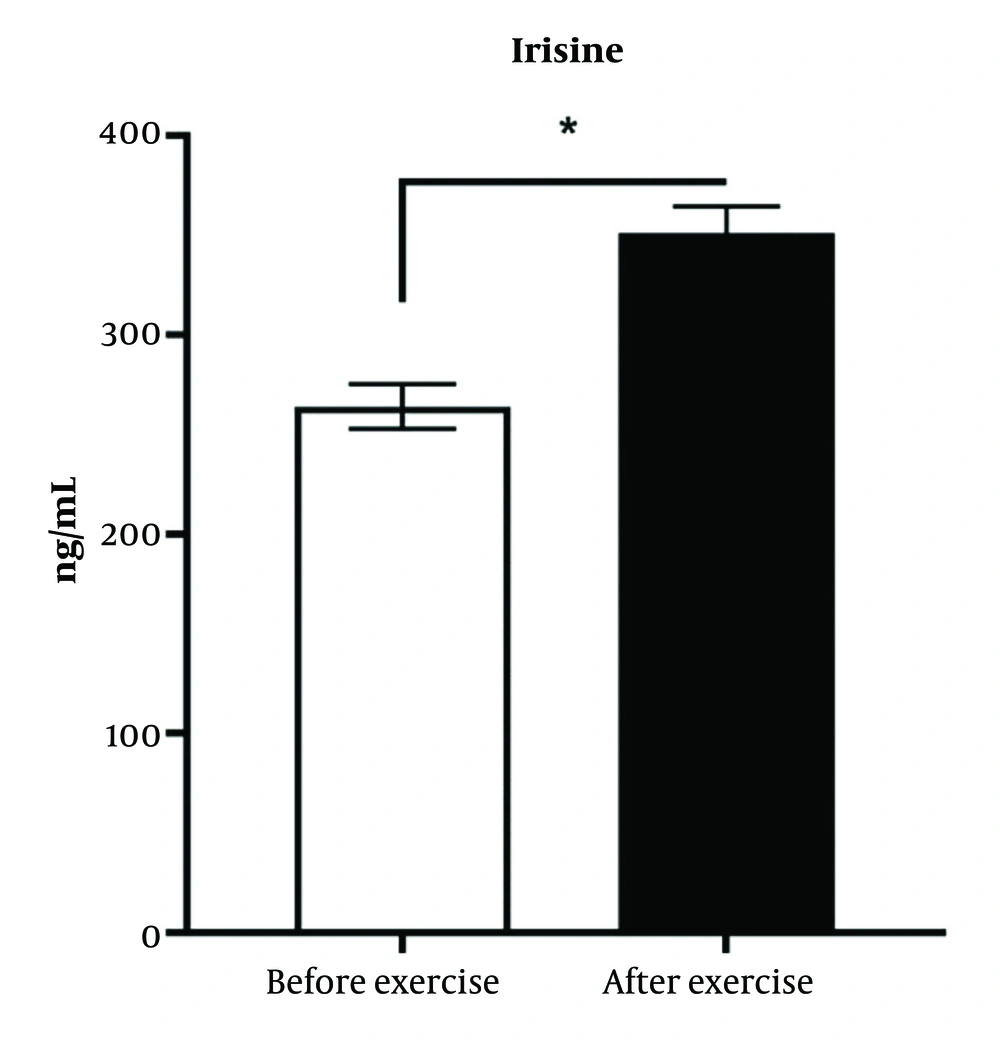
4.6. Effect of High-Intensity Interval Training Intervention on the Serum Level of Irisin Hormone in Diabetic Patients
As shown in Figure 6, it has been demonstrated that training intervention increases the serum level of Irisin hormone 1.42 folds in diabetic patients.
There was a significant difference between the diabetic group and the training intervention group (P < 0.05).
5. Discussion
Training interventions and lifestyle modifications are among the most effective methods for preventing, controlling, and treating metabolic diseases such as diabetes, obesity, and related disorders like cardiovascular diseases and osteoporosis. Recent research conducted by Omidifar et al. demonstrated that exercise interventions, particularly HIIT, can improve complications of non-alcoholic fatty liver disease (NAFLD) in the liver tissue of diabetic rats induced by a high-carbohydrate, high-fat diet, by activating the SIRT1/PGC-1α signaling pathway (31). In a study by Cauza et al., HIIT, compared to CET, significantly decreased HbA1c, blood glucose, total cholesterol, LDL, and triglyceride levels, while increasing HDL in patients with type 2 diabetes (32). Published papers have shown the beneficial effects of training in improving complications and signs of diabetes in both animal and human models. In this study, the effect of exercise intervention on bone metabolism in diabetic patients was investigated, and the results showed that HIIT increased irisin hormone, osteocalcin, and alkaline phosphatase levels, and decreased CTX levels, which is an important factor in the destruction of bone tissue in diabetic patients, indicating improvement in bone metabolism through training intervention.
5.1. Investigating the Effect of High-Intensity Interval Training on Osteocalcin
In the current study, the findings indicate that the HIIT intervention leads to an increase in osteocalcin levels. This result correlates with the findings of Lester et al., who examined the effects of 8 weeks of aerobic exercise, endurance exercise, and a combination of both on sedentary young women. Their study revealed that osteocalcin levels increased with resistance and combined exercises, although there was no significant effect on parathyroid hormone levels (33). Additionally, Lester et al. conducted a study involving 39 healthy young obese men, where they observed an increase in osteocalcin levels following an 8-week aerobic exercise program (33).
5.2. Investigating the Effect of High-Intensity Interval Training Intervention on Bone Alkaline Phosphatase
High-intensity interval training increases the levels of alkaline phosphatase. This result aligns with the findings of Ooi et al. on hypercholesterolemic women, Zargar et al. on obese men, and Erickson and Vukovich, who also reported an increase in alkaline phosphatase (34-36).
5.3. Investigating the Effect of High-Intensity Interval Training Intervention on the Amount of C-telopeptide
The results indicate a decrease in the amount of CTX in the HIIT group. Erickson and Vukovich conducted a jumping training program on fourteen young adult men over 8 weeks to investigate the effect of jumping exercises on bone markers. The results demonstrated that these exercises reduced the level of CTX (36).
5.4. Investigating the Effect of High-Intensity Interval Training Intervention on the Level of Irisin Hormone
Investigating the effect of HIIT in patients with diabetes has shown that exercise intervention significantly increases the serum level of the irisin hormone. Reisi et al. examined the acute effect of resistance training on irisin protein levels and the expression of muscle FNDC5 and UCP1 genes in male rats, concluding that plasma irisin protein levels increased significantly after a session of resistance training. Additionally, there is a significant increase in the mRNA expression of FNDC5 and UCP1 genes following training (37).
5.5. Conclusions
The study results demonstrate the positive effect of HIIT in improving metabolic markers that affect bone function. The findings indicate that HIIT intervention not only prevents bone loss in patients with diabetes but also enhances protective markers of bone health. Therefore, adopting a lifestyle with high physical activity, in addition to all the beneficial effects on the metabolism of a diabetic person, promotes bone metabolism in favor of increasing bone health.