1. Background
Use of nanoparticles and their oxidative derivatives to overcome bacterial infections - as an antibiotic alternative - has a major role in the elimination of these infections. Nowadays, the use of nanomaterials has developed promptly in all aspects of life, including diagnosis and treatment of diseases (1).
In many studies, antibacterial properties of nanoparticles such as Chromium, Iron, Zink, Silver, and their oxides has been proven (2). Silver, in nano-dimensions, influences the metabolism, respiration, and reproduction of microorganisms. Silver nanoparticles inhibit the bacterial respiratory system without increasing drug resistance (3).
On the other hand, the side effects of chemical drugs have forced scientists to use herbal extracts in the treatment of diseases (4, 5). The indiscriminate use of antimicrobial drugs has been leading to increase drug resistance against different antibiotics in most bacteria. That is one of the reasons for growing use of plants as natural safe, accessible, and affordable materials, than synthetic antibiotics, to eradicate bacterial infections (6).
Seidlitzia rosmarinus is a short shrub with the height of 60 cm to 1.5 m (7, 8). It is highly resistant to alkaline and saline soils and often grows in desert areas such as Dasht-e Kavir and Lut of Iran as a native and adapted species (9). Clinical studies have shown that hydro-alcoholic extract of Seidlitzia rosmarinus, with concentration of less than 15%, has the therapeutic effect on the mouse model of cutaneous leishmaniasis (10). It has been shown that silver nanoparticles (400 ppm), inhibits growth of E. coli (11).
Nowadays, S.aureus is known as a major cause of hospital-acquired infections (12). Since the bacterial resistance plasmids are transposable, pathogenic and drug-resistant strains of this bacterium has increased by up-taking various plasmid genes (13). S.aureus is a Gram-positive and facultative anaerobic coccus and causes a wide range of infections such as osteomyelitis, food poisoning, septicemia, Furuncle, carbuncle and other local or visceral diseases (14). K. pneumoniae is a Gram-negative enteric bacillus and is a member of the Enterobacteriaceae family and form part of the natural microflora of the human body (15, 16). Approximately 1/3 of healthy individuals are intestinal carriers of this germ and, today, these bacteria are discussed as an opportunistic pathogen and 1 of the main bacteria in Hospital-acquired infection (17). The aim of the current study was to evaluate antimicrobial effects of Seidlitzia rosmarinus and silver nanoparticles in the growth inhibition of MDR strains of S. aureus and K. pneumoniae isolated from urinary tract infections.
2. Methods
2.1. Sample Collection and Bacteriologic Identification
A total of 25 clinical isolates of S. aureus and 35 Clinical isolates of K. pneumoniae were collected from Razi medical laboratory of Rasht city and were identified using Gram staining and biochemical tests such as Catalase, Coagulase, Mannitol fermentation, and DNA hydrolysis for S.aureus and Indole, MR-VP, Citrate, H2S production, Motility, Urea hydrolysis, and triple sugar iron Agar (TSI) reactions for K. pneumoniae.
2.2. Detection of MDR Strains
MDR strains of S.aureus were determined by disk diffusion method. For this purpose, susceptibility of S. aureus isolates was detected by Cefoxitin disk (30 µg) on Mueller Hinton Agar (Merck, Germany) and strains having ≤ 21 mm zone of inhibition were considered as MDR strains (18). MDR strains of K. pneumoniae were determined by combination disk method (Cefotaxime (30 µg) alone and with Clavulanic acid (10 µg)) on Mueller Hinton Agar (Merck, Germany). MDR strains were confirmed by ≥ 5 mm increase in inhibition zone of Cefotaxime with Clavulanic acid (19).
2.3. MTT Assay
The cell survival against the concentrations of extract and silver nanoparticle was measured in Hela cell line (20).
2.4. Silver Nanoparticles Preparation
Silver nanoparticles with dimensions of approximately 20 nm were purchased from NanoSany corporation (Tehran, Iran).
2.5. Preparation of Seidlitzia rosmarinus extract
In order for preparation of Seidlitzia rosmarinus extract, about 500 g of this plant was poured into a large flask as a powder and diluted methanol was added to it to a final volume of 1 liter. After 48 to 72 hours, the solution was filtered with Whatman paper. Then, to obtain a pure extract without solvent, the rotary evaporator was used at 40°C under vacuum condition. From concentrated extracts, various concentrations were prepared by DMSO 5% and were used for MIC and disk-diffusion test.
2.6. Determination of MIC and MBC
In order for 0.5 Mc Farland standard preparation, several colonies from fresh and newly cultured bacteria were transferred to Mueller-Hinton broth until the appropriate turbidity of microbial suspension was prepared (0.08 < OD < 0.13). Then, to achieve the turbidity of 1.5 × 108 cfu/mL, the prepared suspension was diluted at a ratio of 0.01. In order to study the antibacterial effect of the herbal extracts, 4 concentrations of 50, 100, 200, and 400 mg/mL of Seidlitzia rosmarinus extract were prepared in 5% DMSO. In the present survey, antibacterial effect of extracts was studied by disk-diffusion and macro dilution methods. In the disk-diffusion, 500 µL of the suspension (1.5 × 108 cfu/mL) was uniformly spread in Mueller-Hinton Agar medium. Then, disks with a diameter of 6 mm and distance of 2.5 cm were put. In the next step, 100 microliters of each of the prepared concentrations were transferred into disks.
All inoculated culture media were placed for 24 hours at 37°C. After a certain period, microbial cultures were examined about the formation or lack of inhibition zone around the disks and the diameter of inhibition zone was measured in mm. Using the macrodilution method, MIC and minimum bactericidal concentration (MBC) were determined.
To determine MIC, dilution series of 6.25, 12.5, 25, 50, 100, 200, and 400 mg/mL of the extract were prepared in Mueller-Hinton broth. Then, 1 mL of the bacterial suspension with a concentration of 1.5 × 106 cfu/mL was added to each dilution. Positive (culture medium containing the bacteria without extract) and negative (culture medium without bacteria) control tubes were also prepared.
In the next step, the inoculated tubes were placed at 37°C for 24 hours. After incubation, the tubes were evaluated in terms of turbidity. The lowest dilution of the extract, without a turbidity (no growth), was considered as MIC. To determine MBC, all tubes of no growth were cultured in Mueller-Hinton Agar medium. Inoculated culture media were incubated at 37°C for 24 hours. The plate with the lowest concentration of the extract and no growth of bacteria was considered as MBC of the concentration (21).
MIC and MBC measurement of colloidal silver nanoparticles was performed in the following method: to perform antibacterial tests, silver nanoparticles with dimension of 20 nm were prepared from NanoSany corporation, and different concentrations including 5, 10, 20, and 80 µg/mL were used. Disk diffusion on Mueller-Hinton Agar and MIC and MBC determination on Mueller-Hinton broth were performed, respectively. Preparing the culture media, bacterial strains and the procedure was similar to the one mentioned above. MIC and MBC of silver nanoparticles for the mentioned bacteria were determined on CLSI protocol (22). All experiments were performed in triplicate.
2.7. Statistical Analysis
Data was analyzed by Two-way repeated ANOVA statistical test using SPSS 20 software and P < 0.05 considered as significant.
3. Results
A total of 5 MDR strains of S.aureus and 6 MDR strains of K. pneumoniae were detected by agar disk diffusion and combination disk methods, respectively.
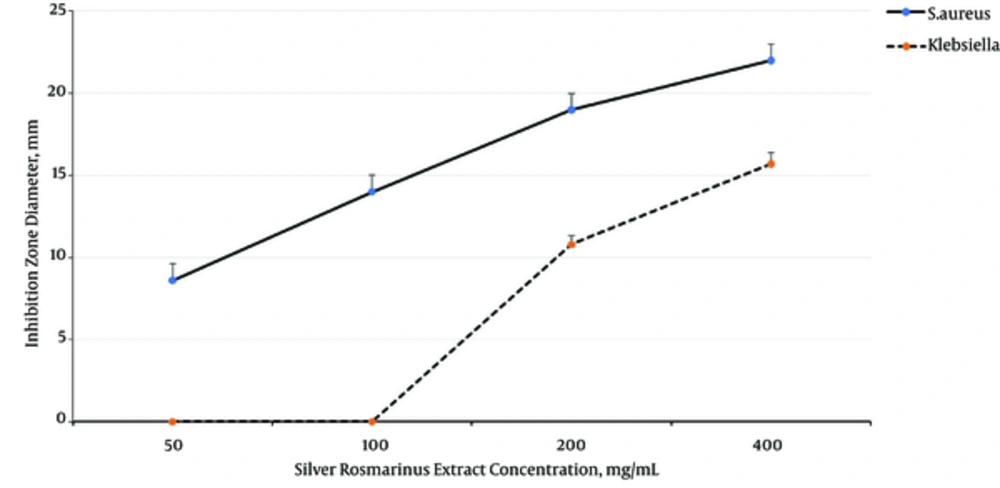
Two-way ANOVA analysis shown that Seidlitzia rosmarinus extract inhibited S.aureus growth (MIC = 100 mg/mL and MBC = 100 mg/mL) more than K.pneumoniae growth (MIC = 200 mg/mL and MBC = 400 mg/mL) (P = 0.001), and inhibited the growth of both bacteria dose dependently (P= 0.001) (Figure 1).
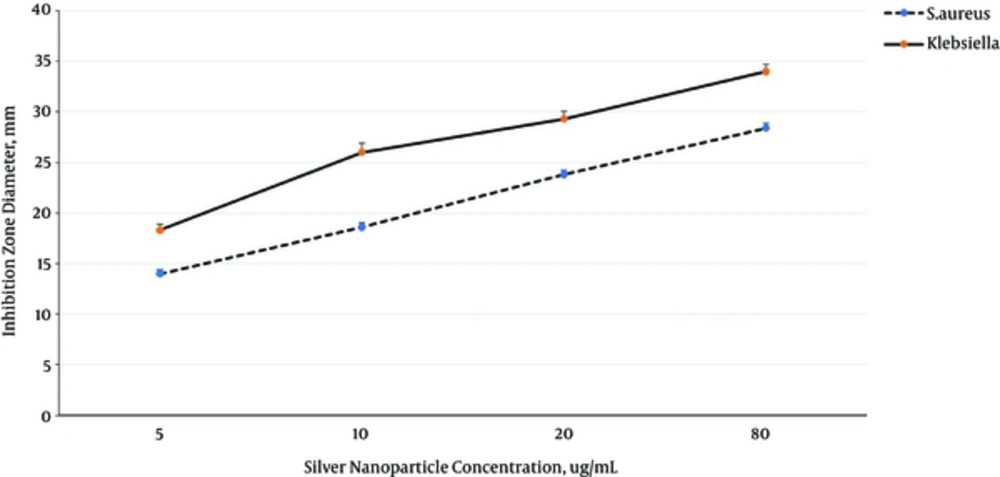
Silver nanoparticles inhibited K. pneumoniae growth MIC = 5 µg/mL and MBC = 10 µg/mL) more than S. aureus growth MIC = 10 µg/mL and MBC = 20 µg/mL) (P = 0.001), and inhibited the growth of both bacteria in dose dependently manner (P = 0.001) (Figure 2).
4. Discussion
Seidlitzia rosmarinus is a therapeutic plant and many studies have demonstrated its properties (23). This plant grows in saline soils and arid deserts. Some reports have suggested that Seidlitzia Rosmarinus confers many medicinal properties and is used in skin and hair disorders as an herbal drug (24). Likewise, stem of this plant has antibacterial and disinfection properties (25).
Silver nanoparticles have strong antimicrobial properties and it is proved to have an antimicrobial effect against a broad range of bacterial species (21). The size of these particles varies between 1 to 100 nm and using them are increasing continually; therefore, they are widely used in the eradication of infections (26, 27). Several studies have been implemented based on potential reaction between the nanoparticles and macromolecules of living organisms. The difference between the negatively charged microorganisms and positively charged nanoparticles acts as an absorber between bacteria and nanoparticles, electromagnetically, and brings about binding of nanoparticles to the cell surface and thus, could lead to cell death (28).
Silver nanoparticles interact with peptide bridges of bacterial peptidoglycan and cause to release muramic acid from peptidoglycan, leading to cell lysis (29, 30). In the present study, it was shown that the antibacterial effect of silver nanoparticles on bacteria is significantly higher than Seidlitzia rosmarinus extract. MIC values of Seidlitzia rosmarinus extract against S. aureus and K. pneumoniae MDR strains were 50 and 200 mg/mL, while MIC values of silver nanoparticles against S. aureus and K. pneumoniae MDR strains were 5 and 10 µg/mL, respectively.
Shirvastava et al. in 2012, concluded that silver nanoparticles are capable to affect against broad spectrum of Gram-positive and Gram-negative bacteria (31). The result of the present study is consistent with the mentioned study.
Kim et al. (2007), and Feng et al. (2000), revealed that the growth of E. coli, in comparison to S. aureus, was inhibited at lower concentrations of silver nanoparticles (27).
Azizian Shermeh et al. demonstrated that the methanolic extract of Seidlitzia rosmarinus has more phenolic and flavonoid compounds than aqueous and ethanolic extracts. In the mentioned study, inhibition zone diameter of S. aureus against 100 mg/mL concentration of Seidlitzia rosmarinus extract was 15 ± 1 mm. These results are consistent with the present study (32).
4.1. Conclusions
It seems that Seidlitzia rosmarinus extract and silver nanoparticles prevent the growth and proliferation of S. aureus and K. pneumoniae in dose dependent manner.