1. Background
Escherichia coli (E. coli), a saprophyte human gut microorganism, can lead to serious infections with resistance to antimicrobial therapies (1). One of the most common infections of this microorganism is urinary tract infection (UTI) (2). Despite the existence of potent antibiotic agents and due to the emerging of multidrug-resistant strains, investigators are focused on the development of new drugs strategies. Natural products often serve as molecules whose activities can be enhanced by manipulation through combinations with chemicals and by synthetic chemistry (3). Plants, as a rich source of secondary metabolites such as tannins, terpenoids, alkaloids, and polyphenols, are generally superior in their antimicrobial activities (3). Furthermore, using medicinal herbs can also lead to prevention of the chemical drug toxicity.
Salvia, commonly called “Kalpoureh” in Iran (4), is a durable, wild-growing, flowering grass plant that can grow to 10 - 30 cm (5). This plant has a callous white exterior and abounds in south-western Asia, Europe, and North Africa (6). The extract of Salvia consists of diterpenoids, 5 - 7- glycoside, thymols, carvacrol, and volatile essences that are found in its aerial parts and has antibacterial activity (7). Due to the synergistic effects of phenolic compounds such as flavonoids, the use of combinations of various Salvia species in traditional antimicrobial therapy is reported in various studies (8).
Rosemary is also another herbal drug that belongs to the Lamiaceae family. There is some evidence from the medical literature that this plant has effective antibacterial components (9).
The main aim of this study was to investigate antimicrobial effects and the synergistic effects of salvia and rosemary with 8 antibiotics against clinical E. coli isolated from urinary samples.
2. Methods
2.1. Plant Material
Specimens of salvia and rosemary officinalis species were collected from the agricultural and natural research center, Kerman, Iran, between April and August 2015.
2.2. Preparation of Plant Extracts
A total of 100 grams of the leaf and flower of the plants have been dried and the final weight of 50 g was powdered and macerated with a mixture of 480 mL methanol and 120 mL sterile distilled water for 5 days at room temperature in darkness. After evaporation of the solvent under reduced pressure, the respective methanol extracts were collected. The resultant product was concentrated by vacuum distillation. All the extracts were kept in tightly closed dark stoppered bottles under refrigeration (4°C) until used for antimicrobial testing (10).
2.3. E. coli Isolation from Clinical Urinary Samples
For three months, from May until July 2016, 680 samples were collected from patients who referred to Aliebn-Abitaleb hospital in Rafsanjan. Accordingly, 60 E. coli isolates were obtained and identified by biochemical tests using indole, methyl red, Voges-Proskauer, citrate (IMVIC), and triple sugar iron agar (TSI) and sulfide-indole-motility (SIM) mediums, as especially mediums, (Biolife Company, Germany). Beta-glucuronidase enzymes test, which is an indicator feature for differentiation from other bacteria, were also used to confirm the bacterium (11).
2.4. Disc Diffusion Test
Qualitative antibacterial activities of the salvia and rosemary extracts as well as Trimethoprim, Ceftriaxone, Cefixime, Cefotaxime, Ciprofloxacin, Gentamicin, Ceftazidime, and Meropenem was evaluated using the Disk diffusion test (Padtan Teb Company, Iran) (12). Accordingly, the data of the antimicrobial susceptibility pattern of the E. coli isolates to commonly-used antibiotics in urinary tract infections were collected, alone and in combination with salvia and rosemary extracts. The concentration of the antibiotics were as follow: Trimethoprim (5 µg/mL), Ciprofloxacin (5 µg/mL), Ceftazidime (30 µg/mL), Ceftriaxone (30 µg/mL), Cefotaxime (30 µg/mL), Meropenem (10 µg/mL), Gentamicin (10 µg/mL), and Cefixime (5 µg/mL) (Padtan Teb Company, Iran) (13). To determinate the suitable concentration of the salvia and rosemary, minimal inhibitory concentrations (MICs) were performed and accordingly, each antibiotic disk was mixed with 1 mL of 32 µg/mL of salvia and 60 µg/mL Rosemary extracts. Both pure antibiotics, salvia and rosemary soaked discs, were incubated in the refrigerator up to 2 hours to stabilize (13). Accordingly, the discs were included 1, salvia; 2, rosemary; 3, salvia plus antibiotics; and 4, rosemary plus antibiotics impregnated disks. Additionally, the MIC was calculated for each group.
Approximately, 108 bacteria strains were cultured as 0.5 McFarland’s concentration using a sterile swab, and then the antibiotic discs impregnated with Salvia and Rosemary’s officinalis extractions with forceps sterile and then was transferred to the plate containing Muller Hinton Agar and standard inoculums of bacteria. Inhibition zone was measured after 18 hours based on the CLSI guidelines for antimicrobial susceptibility testing (14). Accordingly, data were presented as percent of sensitive and resistance E. coli to the antibiotics. However, Salvia and Rosemary synergistic effects may be at sensitive regions, hence, inhibition zones are also reported in the results section to show the synergistic effects.
2.5. Serial Dilution Assay
The MIC of the extracts was determined using broth microdilution techniques as described by the national committee for clinical laboratory standards in microtiters of 96 wells. Then, MIC values were determined in Mueller Hinton Broth (MHB), which is prepared from Biolife Company, Germany, by incubation overnight at 37°C for 24 hours (15). Extracted stock solutions were two-folds diluted from 0.5 to 1024 micro gr/mL (final volume = 80 µL) and a final Dimethyl sulfoxide (DMSO) concentration ≤ 1%. Then, 100 µL of MHB were added onto microplate wells. Finally, 20 µL of 106 colony forming units (CFU/mL) (according to McFarland turbidity standards) of standardized bacterial suspensions were inoculated onto microplate wells and the test was performed in a volume of 200 µL. Plates were incubated at 35°C for 24 hours. The same tests were performed simultaneously for growth (MHB + bacteria) and sterility controls (MHB + extract). The MIC was calculated as the highest dilution showing complete inhibition zone of tested strain. Only extracts that showed antimicrobial activities from agar-well diffusion method were tested for MIC.
2.6. Data Analyze and Statistical Methods
The data were analyzed using SPSS18 software and, Chi-square, Fisher, ty Anova statistical tests. The level of significance was considered P < 0.0 5 in all tests.
3. Results
The MIC of salvia and rosemary extract on 60 E. coli clinical isolates were 32 and 64 µg/mL, respectively. Thus, the concentrations were used for evaluation of the synergistic effects of salvia and rosemary on the antibiotics against all E. coli isolates. MIC of the antibiotics was also calculated and Figure 1 shows a sample.
The results revealed that E. coli sensitivities to Trimethoprim, Ciprofloxacin, Ceftazidime, Ceftriaxone, Cefotaxime, Meropenem, Gentamicin, Cefixime, Salvia, and Rosemary were 36.7%, 80.0%, 46.7%, 63.3%, 63.3%, 100.0%, 83.3%, 83.3%, 60.0%, and 58.3%, respectively. Experimental studies also demonstrated that using Salvia extract with Trimethoprim, Ciprofloxacin, Ceftazidime, Ceftriaxone, Cefotaxime, Meropenem, Gentamicin, and Cefixime altered E. coli sensitivities to 56.7%, 86.7%, 93.3%, 73.0%, 70.0%, 100.0%, 86.7%, and 76.7%, respectively. Additionally, Rosemary also altered E. coli sensitivities to Trimethoprim (60.0%), Ciprofloxacin (86.7%), Ceftazidime (93.3%), Ceftriaxone (76.7%), Cefotaxime (63.3%), Meropenem (100.0%), Gentamicin (86.4%), and Cefixime (76.3%). Table 1 shows the clinical isolates of E. coli sensitivities to the antibiotics either alone or in combination with salvia and rosemary.
| SXT and RM | SXT and SV | |||||
|---|---|---|---|---|---|---|
| Sensitive | Resistance | P Value | Sensitive | Resistance | P Value | |
| SXT | 0.604 | < 0.001 | ||||
| Sensitive | 23 (71.9) | 11 (64.7) | 33 (100) | 3 (15.8) | ||
| Resistance | 9 (28.1) | 6 (35.3) | 0 (0) | 16 (84.2) | ||
| SXT and RM | - | 0.682 | ||||
| Sensitive | - | - | 22 (68.8) | 12 (63.2) | ||
| Resistance | - | - | 10 (31.3) | 7 (36.8) | ||
| CRO and RM | CRO and SV | |||||
| Sensitive | Resistance | P value | Sensitive | Resistance | P value | |
| CRO | < 0.001 | < 0.001 | ||||
| Sensitive | 36 (97.3) | 9 (60) | 39 (97.5) | 7 (53.8) | ||
| Resistance | 1 (6) | 6 (40) | 1 (2.5) | 6 (46.2) | ||
| CRO and RM | - | 0.262 | ||||
| Sensitive | - | - | 31 (70.5) | 7 (53.8) | ||
| Resistance | - | - | 13 (29.5) | 6 (46.2) | ||
| CFM and RM | CFM and SV | |||||
| Sensitive | Resistance | P value | Sensitive | Resistance | P value | |
| CFM | 0.007 | 0.086 | ||||
| Sensitive | 36 (78.3) | 2 (28.6) | 32 (80) | 6 (54) | ||
| Resistance | 10 (21.7) | 5 (71.4) | 8 (20) | 5 (45.5) | ||
| CFM and RM | - | < 0.001 | ||||
| Sensitive | - | - | 42 (100) | 6 (42.9) | ||
| Resistance | - | - | 0 (0) | 8 (57.1) | ||
| CTX and RM | CTX and SV | |||||
| Sensitive | Resistance | P value | Sensitive | Resistance | P value | |
| CTX | 0.790 | 1.0 | ||||
| Sensitive | 38 (79.2) | 6 (75.7) | 46 (100) | 0 (0) | ||
| Resistance | 10 (20.8) | 2 (25 ) | 0 (0) | 14 (100) | ||
| CTX and RM | - | 0.790 | ||||
| Sensitive | - | - | 38 (86.4) | 10 (83.3) | ||
| Resistance | - | - | 6 (13.6) | 2 (16.7) | ||
| CP and RM | CP and SV | |||||
| Sensitive | Resistance | P value | Sensitive | Resistance | P value | |
| CP | 0.094 | 1.0 | ||||
| Sensitive | 46 (95.8) | 6 (75) | 52 (100) | 0 (0) | ||
| Resistance | 2 (4.2) | 2 (25) | 0 (0) | 4 (100) | ||
| CP and RM | - | 0.034 | ||||
| Sensitive | - | - | 46 (88.5) | 2 (50) | ||
| Resistance | - | - | 6 (11.5) | 2 (50) | ||
| GM and RM | GM and SV | |||||
| Sensitive | Resistance | P value | Sensitive | Resistance | P value | |
| GM | ||||||
| Sensitive | 26 (92.9) | 4 (40) | 0.002 | 52 (100) | 0 (0) | 1.0 |
| Resistance | 2 (7.1) | 6 (60) | 0 (0) | 8 (100) | ||
| GM and RM | - | 1.0 | ||||
| Sensitive | - | - | 26 (86.7) | 2 (25) | ||
| Resistance | - | - | 4 (13.3) | 6 (75) | ||
| CAZ and RM | CAZ and SV | |||||
| Sensitive | Resistance | P value | Sensitive | Resistance | P value | |
| CAZ | ||||||
| Sensitive | 56 (93.3) | 56 (93.3) | 1.0 | 56 (100) | 0 (0) | 1.0 |
| Resistance | 4 (6.7) | 4 (6.7) | 0 (0) | 4 (100) | ||
| CAZ and RM | - | 1.0 | ||||
| Sensitive | - | - | 56 (100) | 4 (100) | ||
| Resistance | - | - | 0 (0) | 0 (0) | ||
aValues are expressed as No. (%).
bThe Table shows that Rosemary (RM) converts sensitivities of E. coli to Cefixime (CFM) and Gentamicin (GM) and Salvia (SV) significantly increased resistance of E.coli to Trimethoprim (SXT) and Ceftriaxone (CRO). Salvia and Rosemary had not affect sensitivities of E. coli to Cefotaxime (CTX), Ciprofloxacin (CP) and Ceftazidime (CAZ).
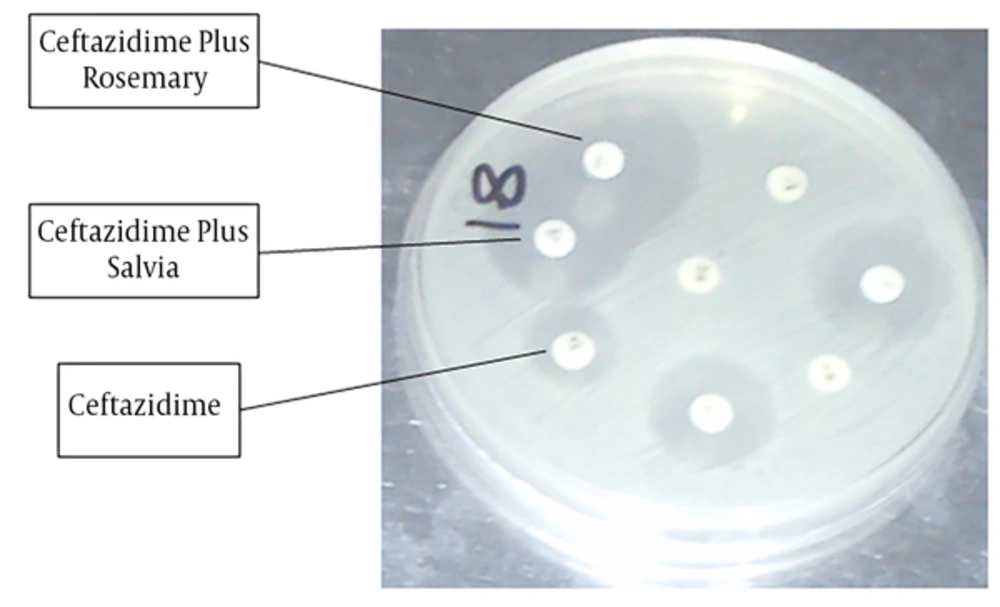
The results revealed that the inhibition zone in Ceftazidime, Ceftazidime plus rosemary, and Ceftazidime plus salvia were, 17.50 ± 0.43, 24.11 ± 0.79, and 24.13 ± 0.81, respectively (Figure 2). Data analysis showed that the differences between Ceftazidime and Ceftazidime plus rosemary (P < 0.001) and also between Ceftazidime and Ceftazidime plus salvia (P < 0.001) were significant. Data analysis also revealed that the differences regarding zone of inhibition in Ceftriaxone versus Ceftriaxone plus rosemary (P = 0.005) was significant and Ceftriaxone plus rosemary increased the inhibition zone.
However, data analysis revealed that other competitions were not significant. Accordingly, the results revealed that the inhibition zone in salvia was 5.53 ± 0.71 and in rosemary was 5.48 ± 0.91 (P = 1.0). The results also showed that inhibition zone in Trimethoprim, Trimethoprim plus rosemary, and Trimethoprim plus salvia were, 14.13 ± 0.78, 17.65 ± 1.01, and 16.06 ± 1.30, respectively. Data analysis showed that there were no significant differences between trimethoprim and trimethoprim plus Rosemary (P = 0.429) and also between Trimethoprim and Trimethoprim plus Salvia (P = 0.997).
Data showed that there were no significant differences in ceftriaxone versus ceftriaxone plus salvia (P = 0.307), Cefixime versus Cefixime plus rosemary (P = 1.00), Cefixime versus Cefixime plus salvia (P = 0.999), Cefotaxime versus Cefotaxime plus rosemary (P = 0.985), Cefotaxime versus Cefotaxime plus salvia (P = 0.968), Ciprofloxacin versus Ciprofloxacin plus rosemary (P = 1.0), Ciprofloxacin versus Ciprofloxacin plus salvia (P = 1.0), Gentamicin versus Gentamicin plus rosemary (P = 0.983) and also Gentamicin versus Gentamicin plus salvia (P = 0.983).
4. Discussion
Investigation of the antibacterial properties of indigenous medicinal plants could have a benefit for the development of new drugs to control the growth of an important bacterial pathogen. Ethanolic Salvia extract has been shown to have high antibacterial activity against tetracycline-resistant Brucella melitensis (16) E. coli (17) and Salmonella typhi (18), E. coliO157 (19), Pseudomonas aeruginosa, Bacillus cereus, as well as Candida albicans and Aspergillus niger (20). In the present study, the synergistic antibacterial activities of salvia and rosemary with some common antibiotics against E. coli isolated from clinical samples (UTI) have been explored. The results demonstrated that the sensitivities of E. coli to Ceftazidime significantly increased after using rosemary (Table 1), but not Salvia. In addition, accordingly, rosemary increased the inhibition zone of the antibiotic significantly. Additionally, either rosemary or salvia also significantly had synergistic effects on the inhibition zone of the Ceftriaxone. It has been reported that either Ceftazidime or Ceftriaxone has antibacterial effects by inhibition of cell wall synthesis (21, 22). Accordingly, Ceftazidime via replacement with peptidoglycan components and Ceftriaxone via binding to the transpeptidases (transaminases), which are penicillin-binding proteins (PBPs), inhibit bacterial cell wall synthesis (21, 22). Based on the results, it appears that rosemary through elevation of Ceftazidime and Ceftriaxone mechanisms increased their effects on E. coli growth. Accordingly, it may be hypothesized that rosemary increased replacement of Ceftazidime in the E. coli cell wall. The synergistic effects of rosemary on the Ceftriaxone may be due to increased antibiotic binding to PBPS. However, based on the fact that rosemary had no synergistic effects on the Cefotaxime and Cefixime, which have similar mechanisms to inhibition of cell wall generation via binding to the PBPs (23, 24), it may be hypothesized that synergistic effects of rosemary on the Ceftriaxone may be related to the chemical components of Ceftriaxone, which needs to be explored by further investigation. Meanwhile, the sensitivity of E. coli to Ceftriaxone was decreases after using Ceftriaxone with rosemary and 9 cases were resistant to Ceftriaxone plus rosemary, while they were sensitive to Ceftriaxone alone. Interestingly, results showed that salvia had synergistic effects on the Ceftazidime only. Based on the main mechanisms used by Ceftazidime against E. coli, inhibition of peptidoglycan synthesis, it appears that salvia has similar effects on the antibiotic like rosemary. However, salvia was unable to increase the inhibition zone on other antibiotics, even Ceftriaxone. Based on the fact that both salvia and rosemary had no synergistic effects on the Gentamicin, Ciprofloxacin, and Trimethoprim, which affects E. coli via inhibition of translation, DNA replication, and folic acid synthesis, respectively (25-27), it may be hypothesized that either salvia or rosemary do not have any synergistic effects on the antibiotics.
The results also demonstrated that rosemary increased E. coli resistance to Cefixime and Gentamicin in 2 and 4 cases, respectively. Salvia induces E. coli resistance to Trimethoprim in 3 cases and Ceftriaxone in 7 cases (Table 1). Based on the fact that salvia and rosemary increased inhibition zone in Ceftazidime only, and had no synergistic effects on other antibiotics (except Rosemary on Ceftriaxone), and based on the negative roles played by salvia and rosemary on some antibiotics and decreased their sensitivities, it seems, that using salvia and rosemary can be considered as a supplementary drug in association with Ceftazidime only and their use in association with other antibiotics needs to be avoided. In parallel with our results, Nascimento et al., also reported that salvia extracts did not present any antimicrobial activities against some antibiotic-resistant bacteria including E. coli (28). In contrast with our results, Kamatou et al., reported the synergistic actions of salvia against the gram-positive bacteria (29). Interestingly, while various results of antagonism, synergism, and/or additive actions were observed on the gram-negative bacteria (29). Based on previous results and our results, it seems that salvia may increase sensitivities of gram-positive to the antibiotics, in spite of gram-negative bacteria, which needs to be evaluated with further investigations.
Due to the fact that all E. coli isolates were sensitivities to either Meropenem alone or in combination with salvia and rosemary, the synergistic effects of the antibiotic have not been evaluated.
4.1. Conclusion
Finally, based on the results, salvia and rosemary have synergistic effects with Ceftazidime against E. coli. Due to the effects of the antibiotic on the E. coli cell wall, it may be concluded that salvia and rosemary increased the main mechanisms used by the antibiotic on the bacteria cell walls and, hence, can be considered as a future therapy against resistant E. coli isolates in UTI.