1. Background
One of the problems encountered in the nervous system may be due to exposure to polyvinyl chloride (PVC), which is used as a raw material in the manufacture of chemical and plastic industries, including disposable containers and plastic tubes, cables and wires, floor coverings, film photography, automotive electronics, and toys (1-3). Due to the increased production of the above-mentioned products, PVC production will also increase, resulting in more exposure to PVC consumers and carriers, which currently has more than 81,000 PVC dealers (4). Since PVC does not chemically bond to the polymer, it is removed at the time of the production and use of the polymer (2, 3). Therefore, it is transmitted to humans through the air, water, food, and even using medical devices (1, 5) and children’s toys (6, 7). One of the most important uses of PVC is in the manufacture of medical devices and laboratory equipment (1). Blood storage bags, injection and hemodialysis devices, and chip tubes contain large quantities of PVC. Many materials used to make PVC industries are found in the blood and tissues of patients, who have a frequent transfusion (8). However, the main source of human exposure to PVC is through food (7). Most studies have been conducted on the effect of PVC on the liver (4). In people exposed to PVC, the risk of developing liver Angiosarcoma is 11 to 16 times higher, and the risk of developing brain cancer in these people is four times higher than that of normal individuals (9). Furthermore, PVC is a ubiquitous environmental toxicant with high exposure potential to males. This environmental agent is found in the air, drinking water, and food (10). The recent research showed that environmental contaminants, like PVC, have been implicated in some neuropathological conditions. Vitamin E is an antioxidant substance that prevents memory disturbance (11, 12), such as mental disorder (13), diabetes (14), cerebral stroke (15), Alzheimer’s disease (16, 17), and aging (18).
2. Objectives
The present study examined the protective effect of vitamin E against the damage caused by exposure to PVC in the rat hippocampus.
3. Methods
3.1. Experimental Protocol
This study used 24 adult male Wistar rats (weighing 180 to 200 g). Animals were carried out in accordance with the Animal Ethical Committee Acts of Urmia University. All animals were housed in a cage with free access to water and food. The temperature of the room was 21°C and 65% humidity with 12-hour light/dark cycle.
3.2. Animals
Rats were randomly assigned to four groups (n = 6). Groups included control, vitamin E (150 mg/kg bw per day), polyvinyl chloride (1000 mg/kg bw per day), and the combination group. The administration route was oral, and the experimental period was 40 days. Vitamin E and PVC (Merck, Germany) were dissolved in normal saline solution and olive oil, respectively (19).
3.3. Tissue Preparation
After a period 40 days, rats were anesthetized and sacrificed. Then, in the sterile conditions, the brain hemispheres were removed. For tissue evaluation, the left hemisphere was placed in 10% fixative buffered formalin solution and then underwent brain processing and paraffin embedding. Paraffin-embedded tissues were sliced to 5-μm thick sections using a rotary microtome (Germany, serial: 21074) and transferred to slides. Finally, the prepared slides were stained using Hematoxylin-Eosin, TUNEL, and crystal fast violet methods.
3.4. Nissl Staining
To evaluate histological changes, cresyl fast violet or nissl staining was performed. Sections were de-paraffinized in xylene, rehydrated, and saturated in 0.1% cresyl violet acetate solution for 10 minutes. Sections were differentiated in 95% ethanol, dehydrated, cleared, mounted, and then analyzed under light microscopy (20).
3.5. TUNEL Staining
DNA fragmentation and apoptotic cells were visualized by TUNEL staining method. Briefly, the dewaxed (using two changes of xylene) brain tissue sections were predigested with 20 mg/mL proteinase K for 20 minutes and then incubated in a phosphate-buffered saline solution (PBS), containing 3% H2O2 for 10 minutes in order to block the endogenous peroxidase activity. The sections were thereafter incubated with the TUNEL reaction mixture, fluorescein-d UTP (In situ Cell Death Detection kit, Fluorescein, Roche, Germany), for 60 minutes at 37°C, based on the manufacturer’s instructions. The slides were then rinsed three times with PBS (three times). Thereafter, Hoechst stain (33258, Sigma-Aldrich) for the chromogenic reaction was added to the sections and using the TUNEL reaction was omitted in negative control sections.
3.6. Histological Studies
For hippocampus evaluation, the numerical density of neurons in CA1, CA2, CA3, and Dentate Gyrus (DG) regions were estimated using a light microscope count and the apoptotic index was calculated (21).
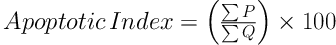
Where the ΣP is the sum of the number of apoptotic cells and ΣQ is the sum of total cells.
3.7. Statistical Analysis
Data were analyzed using SPSS 24. Groups were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post-hoc test. Significance level was considered as P < 0.05.
4. Results
4.1. Histological Results
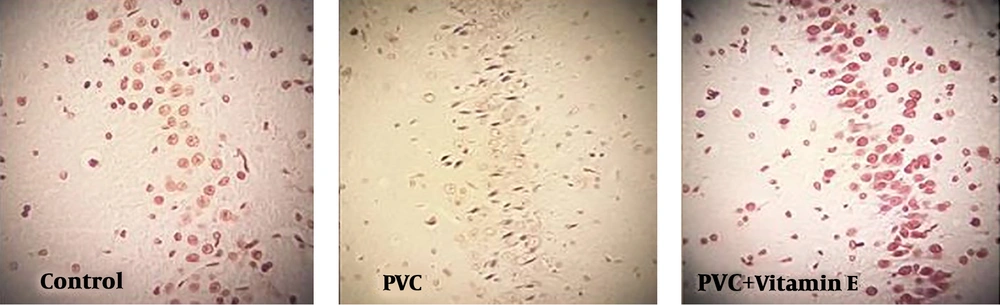
The effects of PVC and vitamin E treatment on the apoptotic index is summarized in Table 1 and Figure 1. The CA1 apoptotic index of PVC-treated group was significantly higher than control and vitamin E groups (P < 0.05), although there were no significant differences between the control group and vitamin E treated group (P > 0.05). In the combined group, the apoptotic index was significantly lower than the PVC group, however, there was a significant difference than the control group (P < 0.05).
| Groups | Apoptotic Index in the Experimental Groups | |||
|---|---|---|---|---|
| CA1 | CA2 | CA3 | DG | |
| Control | 13.20 ± 0.93 | 10.17 ± 0.93 | 11.85 ± 1.14 | 17.35 ± 0.7 |
| Vitamin E | 10.69 ± 1.66 | 8.70 ± 0.50 | 10.26 ± 0.30 | 14.88 ± 0.91 |
| PVC | 38.91 ± 1.66b | 18.96 ± 0.87b | 26.41 ± 3.29b | 30.96 ± 4.67b |
| PVC + vitamin E (combined) | 28.76 ± 1.56b | 13.51 ± 1.29b | 18.48 ± 1.60b | 24.26 ± 1.89b |
aData are expressed as mean ± SE.
bIndicated a significant difference (P < 0.05) compared to the control and vitamin E groups, respectively, in each column.
The CA2 apoptotic index increased in the PVC group (P < 0.05), yet the apoptotic index in vitamin E and combined groups were similar to the control group (P > 0.05).
In comparison to the control group, the CA3 apoptotic index in PVC group was significantly higher than the other groups (P < 0.05). Nevertheless, no substantial differences were obtained in the vitamin E group (P > 0.05). The apoptotic index in the combined group had no significant differences with the control, vitamin E, and PVC groups (P > 0.05).
Similarly, dentate gyrus apoptotic index in the PVC group was significantly higher than the other groups (P < 0.05), yet no substantial differences were found between the vitamin E and control groups (P > 0.05). Also, administration of vitamin E decreased DG apoptotic index in the combined group compared with the PVC group (P > 0.05).
Hematoxylin and Eosin stained sections showed severe degenerative changes and shrinkage cytoplasm and extensively dark picnotic nucleus in the PVC group. In the PVC + vitamin E group, less degenerative changes and slightly shrunken hippocampus was detected.
5. Discussion
The present study demonstrated that exposure to PVC increased apoptosis in cells in the hippocampus and dentate gyrus. The hippocampus, one localized brain region that shows widespread functional and structural plasticity changes during developmental periods and adulthood. The hippocampus has long been studied for its prominent role in learning and memory (22). A number of neural changes have also been reported following PVC exposure in rats. Adult rats exposed to PVC or PVC metabolites showed a significant reduction in the Na+/K+ - ATPase activity that coincided with neuronal degeneration (8).
Exposure to PVC may be considered to trigger a pathological increase in receptor activity and subsequently, reactive oxygen species (ROS)-related excitotoxic damage. Alternatively, PVC may potentiate the toxic effects of ROS via the inhibition of the expression and synthesis of antioxidant enzymes or radical scavengers (10). In the current study, the apoptotic neurons observed in the hippocampus were probably caused by one or several mechanisms mentioned earlier. The authors reported that oxidative stress played a crucial role in PVC-induced brain damage (23). It is known that, like other cells, neurons can protect themselves against excitotoxic and oxidative insults (24). Thus, in general, studies have been focused on neurons in the central nervous system to evaluate the antioxidant defense system of the brain (11, 16, 17, 21, 24-27). On the other hand, it is obvious that glial cells express a variety of neurotrophic factors and cytokines that protect neurons from reactive oxygen species-induced neurotoxicity. Astrocytes are also known to have more antioxidant capacity than neurons. Thus, they protect neurons and promote neuronal survival (28). Due to oxidative stress associated with PVC, enhanced formation of reactive oxygen and ROS occurs; this contributes to increased neuronal death by damaged DNA and augmented levels of lipid peroxidation products in cellular membrane (14). Also, it has been reported to exhibit low endogenous levels of vitamin E (an important biochemical antioxidant) in the brain (29). This could justify why the hippocampus was examined as vulnerable to the consequences of PVC in the current study.
The most important finding of the present study was the protective effects of vitamin E on PVC-induced apoptosis in the hippocampus. In the recent years, the protective effects of vitamin E for oxidative stress have been suggested in some organs (12). Recently, it was demonstrated that vitamin E supplementation decreased alcohol-induced oxidative stress and apoptosis in developing hippocampus and cerebellum (24). Studies demonstrated that, in addition to its direct protective effects on neurons, vitamin E also had beneficial effects on glial cell lines against chemical and/or metabolic insults to the brain. Its protective effects on nerve tissues are scavenging free radicals and stabilizing neuronal and glial cell membranes (28). The results indicate that vitamin E pretreatment may decrease apoptosis and protect the neurons from PVC-induced damage.