1. Background
Osteoporosis as a health problem influences bone mass and structure, which increases the susceptibility of bones to fracture (1). This disease is more common among females, especially post-menopausal women (2). Recently, various drugs, such as bisphosphonates, are applied to treat osteoporosis (3). These drugs usually decrease bone remodeling due to their effect on osteoclast activity and down regulation of osteoclasts differentiation.
Bisphosphonates are among the most broadly recommended drugs in the treatment of osteoporosis since they can prevent osteoclast activity and block bone resorption (4). It has been shown that bisphosphonates induce differentiation of osteoblasts and thereby increase bone formation (4). Zoledronic acid is a nitrogen-containing bisphosphonate that is usually applied intravenously. This bioactive molecule interrupts osteoclastic activity by hindering farnesyl diphosphate (5). Previous investigations represented the beneficial impacts of zoledronic acid application in treating osteoporosis, myeloma, and metastatic bone cancers (6-8). Previous studies indicated that alendronate, an aminobisphosphonate, enhances BMD, reduces bone turnover, and consequently the risk of bone fractures (9). Risedronate, a heterocyclic orally active aminobisphosphonate, has a significantly lower binding affinity with hydroxyapatite crystals compared to zoledronic acid and alendronate (10). This lower binding affinity suggests that at saturation, attachment of hydroxyapatite surface to risedronate occurs to a lesser extent than other bisphosphonate drugs, which gives risedronate the ability to improve BMD and quality of bone, without interfering with antiresorptive activity (10, 11).
Other studies have also investigated the beneficial effects of these drugs on BMD of trabecular bones, however, their effect on different locations (vertebra, hip, and femur), their bone mass, and thickness has not been well investigated.
2. Objectives
Therefore, the current investigation aimed at comparatively evaluating the impacts of zoledronic acid, alendronate, and risedronate on trabecular and cortical bone tissues (hip, femur and vertebrate) in OVX-induced osteopenia animal models.
3. Methods
3.1. Animal Study
A total of 30 adult female Sprague-Dawley rats (225 + 25 g, 10 to 11 weeks) were purchased from the Razi Institute, Karaj, Iran. The animals received ad libitum access to standard chow pellets and water throughout the experimental period.
3.1.1. Experimental Groups and Treatments
The animals were divided to five equal groups as below:
(1) Sham, (2) untreated OVX animals, (3) ZA treated OVX animals, (4) AL treated OVX animals, (5) RI treated OVX animals.
3.1.2. Ovariectomy Procedure
For general anesthesia in rats, 40 mg/kg Ketamine hydrochloride (Woerden, Netherland) and 2 mg/kg Xylazine (Woerden, Netherland) were intramuscularly injected for OVX surgery. Postoperative analgesic [subcutaneous administration of 1 mg/kg Meloxicam (Razak Co., Tehran, Iran)] and antibiotic (5% Enron, Irfan, Tehran, Iran) were also used for five days. Three animals from the sham and OVX group were euthanized at three months post-OVX surgery and their bones were histologically assessed in order to establish the osteoporotic models.
3.1.3. Route and Time of Administration of Drugs
The rats received Phosphate Buffered Saline (PBS) [negative control, (n = 6)], Alendronate (AL, n = 6) (3 mg/kg, PO, daily), Zoledronic acid (ZA, n = 6) (100 µg/kg I.V. single dose), and risedronate (RI, n = 6) (10 µg/kg, SC-2 times/week) for three weeks. Another group was also operated (without ovariectomy) and received PBS daily (sham group) (n = 6) (12). The therapeutic effects of different bisphosphonates were evaluated at three months after OVX surgery, according to previous studies (13).
3.1.4. Euthanasia
Eight weeks post-treatment, the animals were anesthetized by injection of 50 mg/kg ketamine and 2 mg/kg Xylazine (IM), and then euthanized by intra-cardial injection of 1 mg/kg Gallamine triethiodide (Specia, Paris, France).
All animals received humane care in compliance with the Guidelines for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1985). This experiment was approved by the local Ethics Committee of “Regulations for using animals in scientific procedures” in AJA University of Medical Scineces, Tehran, Iran.
3.2. Biochemical Analysis
Five milliliters of whole blood was taken from the heart of animals under deep terminal anesthesia by cardiac puncture. Serum samples were extracted and then stored at -20°C till measurements. The hematocrit (%) and the levels of total protein (TP), Alkaline Phosphatase (ALP), phosphor and calcium were determined by commercially available kits (Pars Azmoon kit, Karaj, Iran). The level of C-telopeptide (CTX), was also determined by Serum CrossLaps One Step ELISA kit (Bio Tech, Denmark) after eight weeks post-treatment, using an BS-200 auto-analyzer (Mindray, China).
3.3. Micro-Computed Tomography (CT) Testing
Micro-CTs were used to assess the density of mid-shaft and head of the femur as compact and trabecular bones, respectively (70 kVp, 114 µA for 800 ms), using Scanco µCT35 scanner (Scanco, Switzerland). For this reason, 200 slices of interested area were evaluated and the related data, such as bone mineral density (BMD), bone volume/total volume (BV/TV), trabecular bone thickness (Tb.Th), and cortical bone thickness (Ct.Th) were comparatively assessed.
3.4. Histologic Evaluations
Right-femur, lumbar vertebra (L5), and iliac bones were dissected, fixed in 10% neutral buffered formalin solution for 48 hours, and decalcified with 10% EDTA (pH 7.4) for 28 days. The paraffin block of the decalcified specimens were then prepared, and the 5-µm thick sections of these samples were stained with Hematoxylin and Eosin (H&E). A descriptive evaluation was used to assess the bone volume, interconnectivity and thickness of lamella, in each sample. Moreover, the percentage of trabecular (hip, vertebra, femoral head) and compact bone thickness (mid shaft of femoral bone) were measured and analyzed, using Image-Pro Plus®V.6 software (Media Cybernetics, Inc., Silver Spring, USA) as the histomorphometric analysis.
3.5. Quantitative Real Time-Polymerase Chain Reaction Analysis
For this analysis, a lumbar vertebra (L6, fresh-frozen sample) from each group was stored at -70°C, until upcoming assessments. Total RNA from the powdered samples were extracted, using RNeasy Micro kit (Qiagen-74004). Briefly, after isolation of total RNA via trizol (Invitrogen™, Thermo Fisher Scientific, USA) and Qiagen RNA isolation kit (QIAGEN, Valencia, CA), based on the manufacturer’s instructions, extracted RNA was evaluated by NanoDrop 2000c Spectrophotometer (Thermo Scientific NanoDrop Products, Wilmington, Delaware, USA). Then, cDNA was created from 2 mg of total RNA via Superscript II reverse transcriptase (Invitrogen). Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Warrington, Cheshire, United Kingdom), according to manufacturer’s guideline. Reaction conditions comprised of 40 denaturation cycles (15 seconds) at 95°C, following one minute of amplification at 60°C. Cycle threshold (Ct) was determined by Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific, USA). All reactions were performed in triplicate, and the expressions were normalized to that of the housekeeping gene beta-actin. Assessed amplification curves for the reactions were represented by the light cycler (Roche Molecular Biochemicals LightCycler Software®, Version 3.5). The markers of cell phenotype were collagen type 1 (Col1 a1), and Alkaline Phosphatase (ALP), and Osteocalcin (OCN) as the osteogenic markers. The related primers are listed in Table 1.
| Gene | Primer Sequence | Size (bp) | Genebank Code | Annealing Temperature (˚C) |
|---|---|---|---|---|
| Ocn | F: GAGGGCAGTAAGGTGGTGAA | 135 | NM_013414.1 | 60 |
| R: GTCCGCTAGCTCGTCACAAT | ||||
| Alp | F: GCACAACATCAAGGACATCG | 195 | NM_013059.1 | 60 |
| R: TCAGTGCGGTTCCAGACATA | ||||
| Col1a1 | F: GAATATGTATCACCAGACGCAG | 186 | NM_053304.1 | 60 |
| R: AGCAAAGTTTCCTCCAAGAC | ||||
| GAPDH | F: GACTTCAACAGCAACTCCCAC | 652 | NM_017008.4 | 60 |
| R: TCCACCACCCTGTTGCTGTA | 1306 |
Abbreviations: Alp, alkaline phosphatase; Col1a1, collagen type 1 a1; GAPDH, host keeping gene; Ocn, osteocalcin.
3.6. Biomechanical Evaluation
The harvested bone from left-femur and lumbar vertebra (L7) (n = 6) were frozen at -20°C until biomechanical evaluation. The biomechanical analysis was performed as previously described (14). The specimens were subjected to compression (trabecular bone) and bending (compact bone), tested by Enduratec ELF 3200 testing machine and a tensile testing machine (Instron, London, UK). The stiffness (N/mm) and maximum load (N) of each specimen were measured and analyzed based on the load-deformation curve (15).
3.7. Statistical Analysis
Data analyses were done by the GraphPad Prism software, version 6.00 (GraphPad Prism, CA). One-way analysis of variance (ANOVA) with subsequent Tukey post-hoc test was used to compare the quantitative data between the experimental groups.
4. Results
4.1. The Micro-CT Scan’s Results
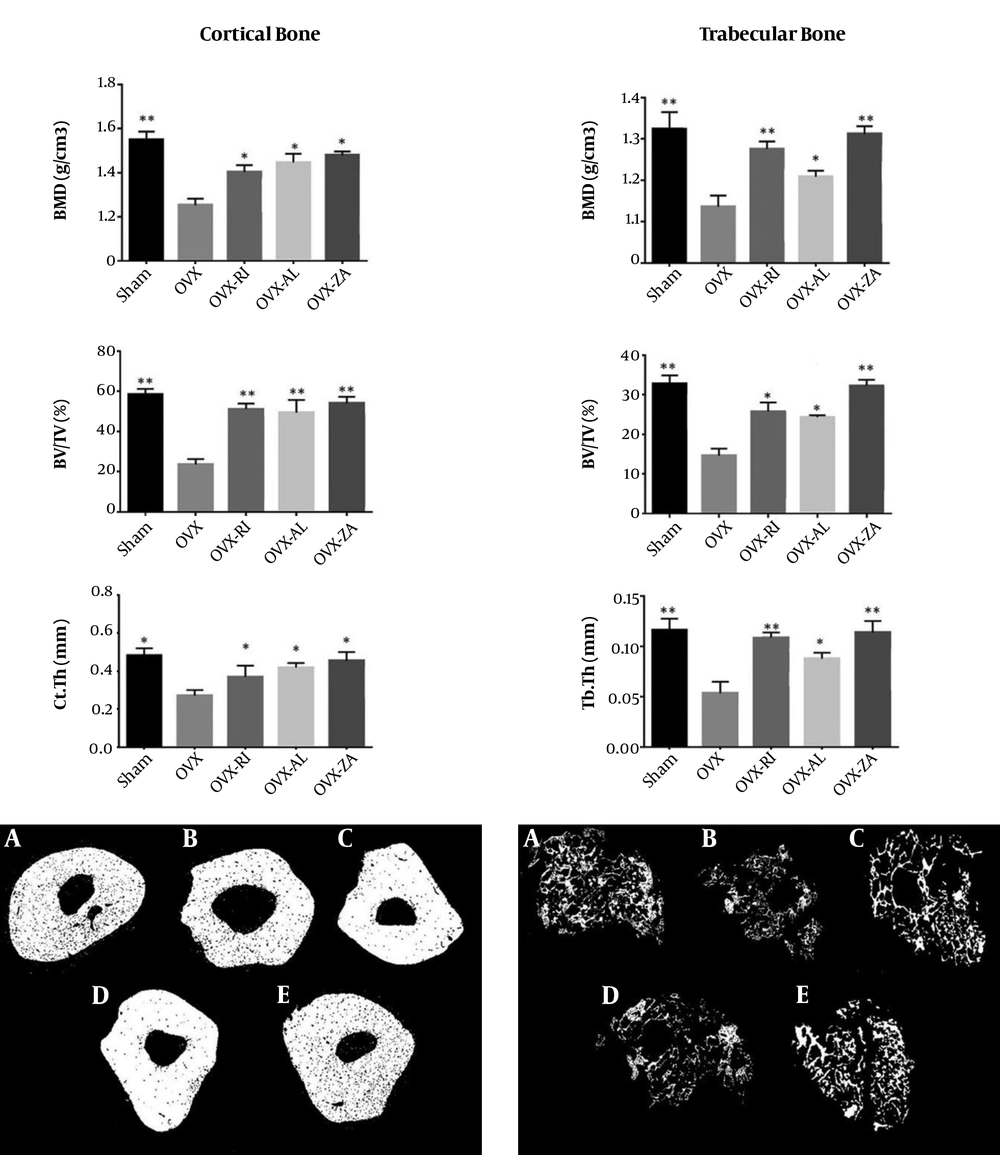
The measurements of BV/TV, Ct.Th, BMD, and Tb.Th of each group at eight weeks post-treatment are presented in Figure 1. The BMDs of cortical and trabecular bones in the ZA, AL, and RI treated groups were significantly higher when compared to the negative control (OVX) (P < 0.05). Among treatment groups, the OVX-ZA group had the highest amount of trabecular and cortical BMD and the measurements did not show a significant difference between the treated group and sham group in terms of compact and trabecular BMD. The rats in the ZA, AL, and RI treated groups showed more BV/TV in comparison to the untreated animals (P < 0.05). The Ct.Th index of the femur in the ZA, AL, and RI treated groups was significantly higher than the negative control group (P < 0.05). Moreover, the Tb.Th index was significantly higher in all treatment groups in comparison to the untreated group (P < 0.05).
Micro CT analysis of different experimental groups 8 weeks after treatment, A, sham; B, OVX (PBS treated ovariectomized rat); C, OVX-RI (risedronate treated ovariectomized rat); D, OVX-AL (alendronate treated ovariectomized rat); E, OVX-ZA (zoledronic acid treated ovariectomized rat), BMD: bone mineral density, BV/TV: bone volume/total volume, Ct/Th: cortical bone thickness, Tb.Th: trabecular bone thickness, *,**: indicates treatment group versus un-treatment OVX group, **P < 0.01, *P < 0.05.
4.2. Blood and Serum Biochemical Parameters
The impact of treatment regimens on blood and serum biochemical parameters are shown in Table 2. The level of serum Calcium (Ca) was considerably lower in the untreated group compared to the sham and treatment groups (P < 0.05). The serum level of CTX was significantly higher in the negative control compared to other groups (P < 0.01). The level of serum ALP was significantly higher in the OVX-untreated group. Although the serum levels of Ca, P, and CTX in the OVX-ZA group was similar to the sham group, there were no significant differences between this group and other treatment groups in terms of serum biochemical parameters (P > 0.05). Moreover, hematocrit and serum levels of total protein and phosphorus did not show statistically significant differences between the groups (P > 0.05).
| Factors | Sham | OVX | OVX-AL | OVX-RI | OVX-ZA |
|---|---|---|---|---|---|
| HCT, % | 48 ± 3 | 45 ± 2 | 44 ± 6 | 45 ± 8 | 45 ± 7 |
| TP, g/dL | 7.1 ± 0.2 | 6.8 ± 0.3 | 7.0 ± 0.5 | 6.7 ± 0.2 | 7.2 ± 0.8 |
| Ca, mg/dL | 9.4 ± 1.4 | 6.1 ± 1.3b | 8.7 ± 1.4 | 9.0 ± 1.8 | 9.2 ± 0.3 |
| P, mg/dL | 7.2 ± 0.3 | 6.6 ± 0.5 | 6.8 ± 0.4 | 6.8 ± 1.1 | 7.1 ± 0.4 |
| ALP, IU/L | 65.8 ± 3.8 | 135.5 ± 14.4c | 98.7 ± 9.6 | 82.3 ± 6.1 | 91.2 ± 5.2 |
| CTX, ng/mL | 34.5 ± 2.4 | 53.1 ± 4.7b | 38.1 ± 1.8 | 40.5 ± 3.5 | 36.3 ± 2.2 |
Abbreviations: OVX-AL, alendronate treated ovariectomized rat; OVX, PBS treated ovariectomized rat; OVX-RI, risedronate treated ovariectomized rat; OVX-ZA, zoledronic acid treated ovariectomized rat.
a Values are expressed as mean ± SD (n = 6), evaluated by Tukey's multiple comparison tests.
b P < 0.05 (treatments versus sham group).
c P < 0.01 (treatments versus sham group).
4.3. Histologic Findings
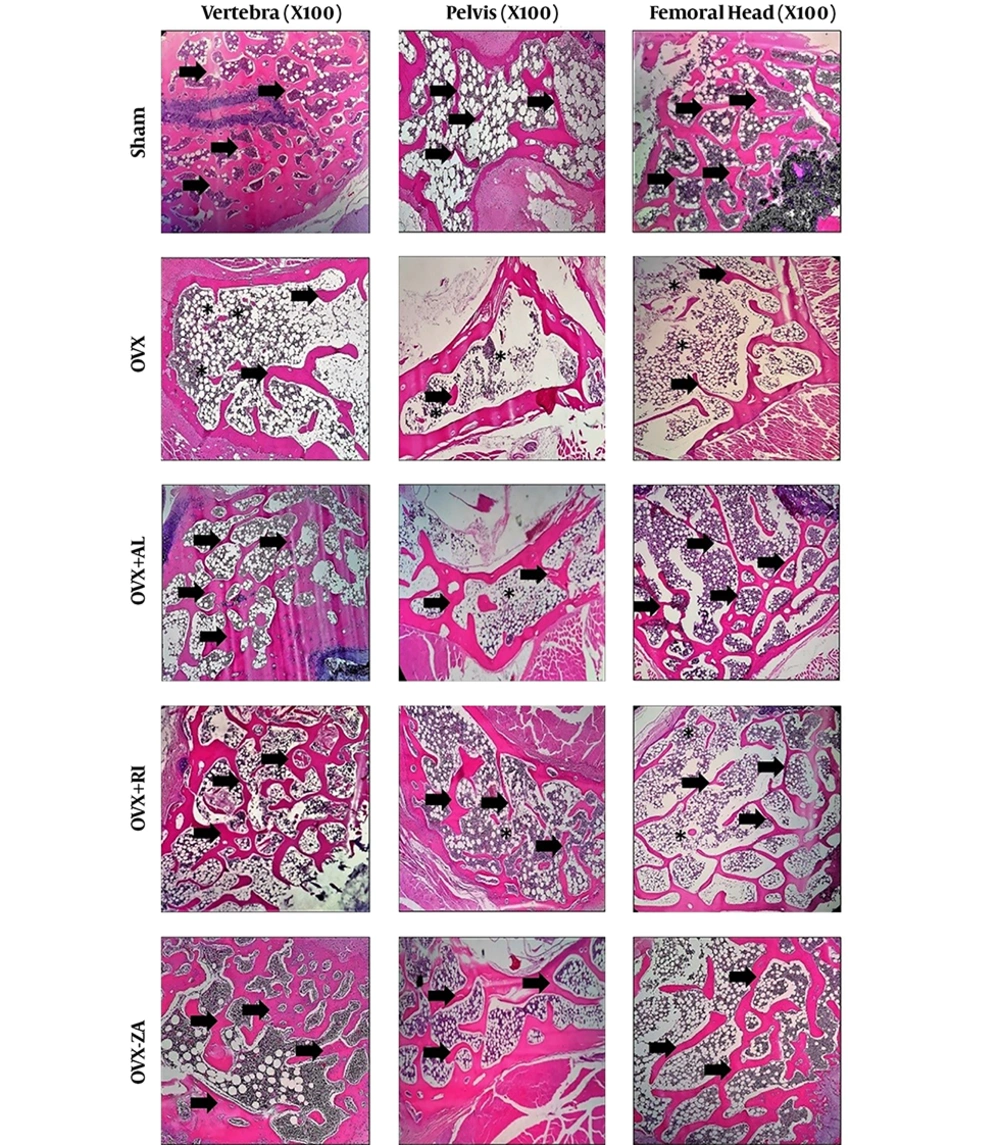
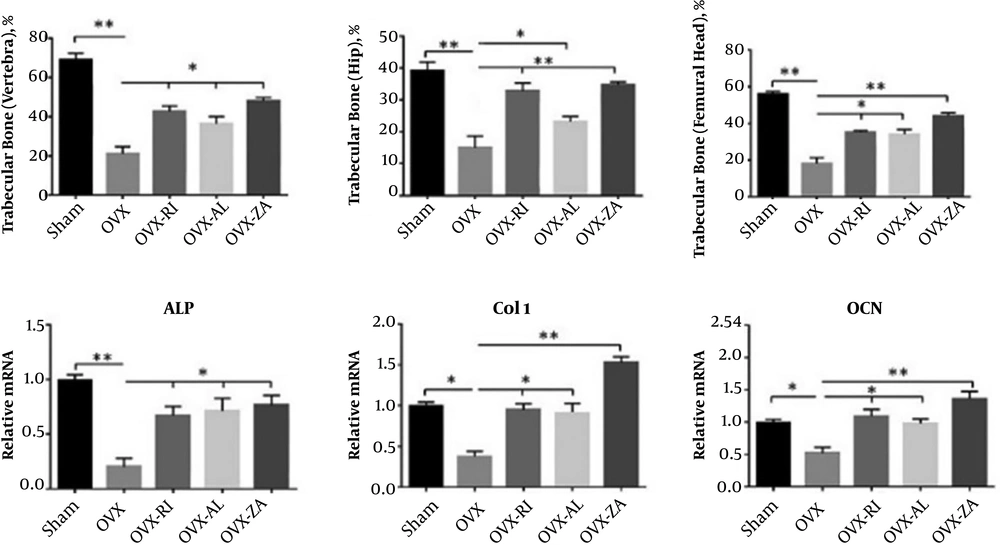
All the tissue sections were comparatively evaluated histopathologically (Figure 2). In addition, the histomorphometric analysis is shown in Figure 3A. The specimens in the sham group showed normal compactness of the vertebral body. The micrographs of lumbar vertebra (L4) in the OVX-untreated group showed thinning of the trabeculae and loss of the trabecular interconnectivity. Trabecular interconnectivity was significantly increased in all treatment groups in comparison to the OVX-untreated group. The micrographs of the hip bone showed a cosiderable reduction in trabecular BMD (%), in the OVX-untreated group, when compared to other groups. In the OVX-untreated group, thinning of trabeculae was also seen in femoral epiphysis. The histomorphometric analysis showed that the trabecular bone of vertebra was significantly increased in all treated groups in comparison to the negative control (P < 0.05). The sham and OVX-ZA groups had a significantly higher femoral trabecular bone (%) when compared to the negative control (P < 0.01), followed by the OVX-AL and OVX-RI (P < 0.05) groups. Histomorphometric analysis of the hip bone micrographs showed that the trabecular bone (%) of sham, OVX-ZA, OVX-RI (P < 0.01), and OVX-AL (P < 0.05) was significantly increased compared to the negative control. Moreover, the hip trabecular bone (%) of OVX-ZA and OVX-RI groups was significantly higher than those of the OVX-AL (P < 0.05) group.
Histopathology section of vertebra, femur, and hip from different experimental groups after 8 weeks post-treatment. Asteroids: loss of interconnectivity, Arrows: trabeculae. OVX: PBS treated ovariectomized rat, OVX-RI: risedronate treated ovariectomized rat, OVX-AL: alendronate treated ovariectomized rat, OVX-ZA: zoledronic acid treated ovariectomized rat.
4.4. Q-RT PCR Analysis
The relative gene expression, including ALP, Col1, and OCN in vertebra were determined by qRT-PCR (Figure 3B). Expressions of the ALP (P < 0.01), Col1, and OCN (P < 0.05) were considerably lower in the OVX-untreated group (NC) in comparison to that of the sham group. Treatment with OVX-ZA, OVX-RI, and OVX-AL prevented a reduction in gene expression levels of the Col1, ALP, and OCN. Moreover, the Col1 expression level was significantly higher in the OVX-ZA group than that of the sham, OVX-AL, and OVX-RI treated groups (P < 0.01).
A, Histomorphometric analysis of all groups including trabecular bone (%) of hip, femoral head, and vertebra 8 weeks post-treatment; B, Effects of different treatments on mRNA expression of osteocalcin (OCN), alkaline phosphatase (ALP), and collagen type 1 (Col1) at 8 weeks post-treatment (vertebral bone). OVX: PBS treated ovariectomized rat, OVX-RI: risedronate treated ovariectomized rat, OVX-AL: alendronate treated ovariectomized rat, OVX-ZA: zoledronic acid treated ovariectomized rat, *,**: indicates treatment group versus un-treatment OVX group ,**P < 0.01, *P < 0.05.
4.5. Biomechanical Performance
The data achieved from the biomechanical testing are available in Table 3. The sham, OVX-ZA, OVX-RI, and OVX-AL groups demonstrated significantly higher maximum load (N) and stiffness (N/mm), for femoral bone, when compared with the negative control (P < 0.05). The femoral and vertebral samples of OVX-ZA group showed higher ultimate load and stiffness in comparison to the OVX-RI or OVX-AL groups, however, these results were not significant (P > 0.05). The stiffness and ultimate load of vertebral bone in the OVX-AL group were not significantly higher than the negative control (P > 0.05). However, all other comparisons of these variables were significantly different (P < 0.05) between the treated and negative control group.
| Value | Sham (1) | OVX (2) | OVX-AL (3) | OVX-RI (4) | OVX-ZA (5) |
|---|---|---|---|---|---|
| Femur | |||||
| Ultimate load, Nb | 44.1 ± 4.2 | 36.2 ± 6.5 | 40.2 ± 4.2 | 41.7 ± 5.7 | 43.2 ± 3.5 |
| Stiffness, N/mmc | 89.3 ± 6.4 | 78.5 ± 3.7 | 84.1 ± 5.1 | 86.9 ± 4.1 | 88.2 ± 2.2 |
| Vertebra | |||||
| Ultimate load, Nd | 125.4 ± 6.6 | 107.1 ± 4.5 | 117.8 ± 5.2 | 120.7 ± 3.2 | 123.6 ± 5.1 |
| Stiffness, N/mme | 1439.4 ± 28.5 | 1371.5 ± 33.7 | 1419.1 ± 24.3 | 1402.5 ± 34.9 | 1430.4 ± 25.2 |
Abbreviations: OVX-AL, alendronate treated ovariectomized rat; OVX, PBS treated ovariectomized rat; OVX-RI, risedronate treated ovariectomized rat; OVX-ZA, zoledronic acid treated ovariectomized rat.
a Values are expressed as median ± SD.
b P < 0.05 (2 vs. 1, 3, 4 and 5).
c P < 0.05 (2 vs. 1, 3, 4 and 5).
d P < 0.05 (2 vs. 1, 4 and 5).
e P < 0.05 (2 vs. 1, 5), (4 vs. 1, 5).
5. Discussion
Osteoporosis is considered as one of the most common disorders among elderly populations, thus, a cost-effective therapeutic or preventive strategy on this disorder should be contemplated. The current study aimed at comparatively evaluating the effects of ZA, AL, and RI on trabecular and cortical bone tissues in an OVX-induced osteoporotic animal model. The radiological, histological, molecular, and mechanical findings showed the best results in the ZA treatment group followed by those of RI.
The OVX rats present one of the best animal models of osteopenia, which clinically mimic the situation of bones in osteoporotic patients (16). Osteoporosis was confirmed by histopathological analysis in ovariectomized rats at three months post-ovariectomy. The results were in agreement with previous investigations that showed a reduced level of calcium and phosphorous in the OVX rat models compared with the sham group (17). In this study, the impact of ZA, AL, and RI on cortical and trabecular bone mass of different bones were assessed in the OVX rats. The study showed that BMD increases at the vertebra, trabecular, and cortical part of the femur and hip bone after treatment by ZA and RI. Changes in the level of bone specific-gene expression and serum biochemical markers during the treatments were consistent with these findings.
The current study utilized ZA at a dose level of 100 µg/kg. The outcomes propose that ideal impacts are steady with the discoveries of Ayan et al. (18). They represented that a single perfusion of 0.1 mg/kg of ZA can ameliorate the rate of bone quality and formation. The results of the current study showed an increase in BMD in vertebral trabecular bone with monotherapy of ZA, AL, and RI (27.5% for ZA, 21.3% for RI and 17.7 % for AL). Yano et al. (19) reported that RI may have a greater impact on improving bone quality than AL, despite the less prominent effect on BMD. Most studies reported ZA as the most potent drug of bisphosphonates, which significantly increase the BMD and reduce the risk of fractures in comparison to other bisphosphonates (20). The current results are comparable to these investigations despite using a much lower dosage protocol.
Both micro-CT scan and histological analysis indicated that combination of ZA and RI considerably increased the BMD of trabecular bones, however, there were no significant differences between the results of the ZA, RI, and AL treated group in terms of the BMD of cortical bones. The results are in agreement with other studies that investigated the effects of different bisphosphonates on BMD of vertebrate and hip bones. In contrast, another study showed that the increases in lumbar spine and total body BMD were higher with AL than with RI (21). These controversial results may be relevant to a different dose, dosage, and route of administration of these drugs between the current study and their investigation. The histomorphometric analysis also showed that ZA and RI possess more beneficial effects on hip trabecular bone density in comparison to AL. Moreover, ZA has a greater impact on the trabecular bone of vertebra when compared to AL and RI, which is in agreement with previously reported findings (22).
In order to evaluate the beneficial impact of ZA, RI, and AL on bone tissue, the serum levels of ALP, Ca, P and CTX were assessed. The serum level of Ca was considerably higher in all treatments in comparison to the negative control. CTX as bone resorption marker decreased in all treatment groups at earlier stages and reached the baseline values again. In comparison to OVX-untreated rat group, the levels of each biochemical marker in the ZA, RI and AL treatment groups was almost comparable to the sham. The osteogenic and anti-resorptive effect of ZA, RI, and AL on OVX rats have previously been investigated by analyzing the serum levels of Ca, P and CTX. These studies have shown that these therapies increased the serum Ca and P levels and reduced the serum CTX levels in OVX animals, which were in agreement with the current results (23). The serum level of ALP in the OVX-ZA, AL, and RI treated groups, in the current study decreased when compared to the untreated OVX animals. Anti-resorptive agents, such as ZA reduce the level of ALP in serum, therefore, these therapies maintain the serum level of ALP at a constant level (24).
The expression level of osteogenic genes, such as ALP, OCN, and Col1 was evaluated by RT-PCR, in the present study, and the results showed significantly higher expression levels of these genes in all treatment groups, especially in ZA therapy. The current results were comparable to those of previous studies (25). The impacts of other bisphosphonates, such as pamidronate at a dosage of 0.01 mg/kg/day on the expression of bone regenerating genes in rats have been assessed by Myoung et al. (26). They exhibited that pamidronate hindered osteoclastic activity and activated osteoblastic genes to emit an inhibitor of osteoclast-related resorption.
There are some limitations related to bisphosphonate-based treatments. Bisphosphonates primarily reach the vascular system of bone tissue when utilized systemically, yet they cannot reach the non-vascularized areas (27). In addition, long-term utilization of bisphosphonate has been related to osteonecrosis (28). The current research showed that application of ZA, AL, and RI by used dose and dosage did not show any osteonecrosis. In addition, Gunes et al. (7) suggested a local application of ZA in order to avoid possible disadvantages of systemic delivery. In the current study, the utilization of three drugs of bisphosphonates could significantly increase the BMD of cortical bones. However, only ZA and RI increased the BMD of trabecular bones, as confirmed by in vitro and in vivo analysis.
In conclusion, the in vivo and in vitro findings of the current study indicated that administration of ZA is more beneficial for bone healing in the OVX-induced osteopenic animals in comparison to treatment by AL or RI. Moreover, based on the evaluated osteogenic markers, both ZA and RI drugs can actively promote bone formation in trabecular bones. However, further investigations must be done to reach evidence-based decision making about these bioactive molecules.