1. Background
The conception of memory along with its entity has not been fully elucidated as the molecular mechanisms underlying the storage of memory, and the physical nature of its trace remained unclear. While numerous efforts have been made to unravel the process of memory storage and function, the only reliable method to detect such phenomena is the monitoring of behavioral changes in animal studies to get a new insight, yet indirect, on the functionality of the memory (1). The Morris water maze (MWM) task is a behavioral test used for the determination of spatial learning in rodents. Spatial learning is assessed through repeated trials and reference memory is monitored via preference for the platform area once the platform is missing (2). The obtained information about our daily experience and the establishment of learning experiments in animal studies have revealed that the formation of the long-lasting memory needs repeated practice (3). Spatial memory formation in the MWM task mainly depends upon the hippocampus function in animals. A growing body of evidence indicates that the hippocampus is vital for the acquisition and retrieval of spatial information (4). Studies have demonstrated that the performance in MWM task is tightly associated with hippocampal synaptic plasticity, as measured via long-term potentiation (LTP), and the function of NMDA. These properties have made the MWM a vigorous and reliable examination (5). Myriad investigations have highlighted that the NMDA receptors, which are present in the brain, are involved in many essential biological functions such as neurotoxicity, neuronal plasticity, as well as memory and learning (6). It should be noted that the memory is constituted of multiple stages, including acquisition, consolidation, and retrieval. However, the separation and characterization of each step are experimentally tricky (7). One of the outcomes of the consolidation paradigm is that the experimental mediation could be implemented in two or three different moments during memory formation and recall. Because the formation procedure is not merely an instant process, three formation phases could be determined, namely acquisition which is known as learning, and consolidation, the unstable phase in which the memory stabilizes, and memory retrieval, the stage of bringing back of the learned task (8). Experimental research has reported that the probe trial performance is able to evaluate the spatial memory of laboratory animals (9). One of the conceivable facets of the memory function is the acquisition stage as some molecules have been attributed to this stage of the memory. One of the popular aspects of the acquisition stage of the memory is the availability of the cellular models of learning, involving various forms of synaptic plasticity such as LTP (10). A number of studies implicated that LTP is supposed to be correlated with learning and memory and, more generally stated, enduring experience-dependent improvement of synaptic transmission (5) Hence, the primary target for excitatory synaptic contact dendritic spines, which are located on the pyramidal neurons, hippocampus, and neocortex. Basically, dendritic spines provide a site of synaptic contact by which the neuronal cells could receive input from another neuron (11). It has been shown that dendritic spine density is increased on pyramidal cells in the Cornu Ammon (CA1) region following of two different spatial memory tasks, the MWM and object placement suggesting that they are morphological substrates for the memory function (12). On the other hand, studies indicate that the effects of androgens on the density of spine synapses located on pyramidal neurons in the CA1 area of the hippocampus in male rats. In the CA1 region, the androgen receptors were mainly located on pyramidal neurons, suggesting a potential target for the testosterone action. Gonadectomy had no considerable effects on the number of CA1 pyramidal cells but decreased the CA1 spine synapse density by nearly 50% in comparison with sham and control groups. The treatment of castrated rats with testosterone propionate the density of spine synapse was increased to levels in comparison to the healthy male rats. A similar increase was observed in synapse density in castrated rats after treatment with dihydrotestosterone (DHT) (13).
2. Methods
2.1. Animals
Male Wistar rats weighing 200 - 250 g were purchased from the Pasteur Institute of Karaj, Tehran, Iran. The male rats were housed before the surgical procedures at 23 ± 2°C and the humidity (50 ± 5%) with a 12-h light:12-h dark cycle.
2.2. Surgical Procedures
Rats were anesthetized with intraperitoneal administration of ketamine and xylazine, Alfasan company, Netherlands (3 mg/100 g and 1 mg/100 g of body weight, respectively) and placed in a stereotaxic instrument. Then, two guide cannula were bilaterally implanted in the CA1 region of the hippocampus at coordinates: AP= - 3.8 mm, ML= ± 2.2 mm from Bregma and D/V= - 2.7 mm from dura (4).
2.3. Microinjection Procedure
The intracerebral injection was administered by means of guide cannula using injection needles connected to 10-μL Hamilton microsyringes by polyethylene tubing. The administration of DMSO (0 μg testosterone, 0.5 μL DMSO/side; 30 min before training) and testosterone (80 μg, 0.5 μL DMSO/side; 30 min before training) was performed (pre-training) over 3 min.
2.4. Behavioral Assessment Apparatus
The MWM task consisted of a dark round pool that was filled with water. A transparent platform was placed 1 cm below the surface of the water at the center of the arbitrarily designed north-east, south-east, south-west or north-west orthogonal quadrant. The pool was located in a specific room specified for behavioral experiments with a constant environment and visual cues on curtains around the pool. The animals were placed in water and positioned to face the wall of the pool, and allowed to find the hidden platform underwater (4). A video camera connected to a tracking device (Ethovison XT, version 7) was placed above the pool to monitor the analysis of each trial and measure the traveled distance, escape latency, and swimming speed.
2.5. Behavioral Assessment
In the hidden platform test, the animals were submitted to a daily session of four trials for four consecutive days. During each trial, ninety seconds were specified for each rat to find the platform positioned at the center of the target quadrant. Rats were allowed to remain there for twenty seconds after each trial; then the next trial started and new starting points changed in each trial by a stochastic process. On the fifth day of the experiment, the platform was removed in order to perform the retrieval learning and memory test and the rats were released from the opposite location and allowed to swim freely for sixty seconds. In the visible platform test, the platform was elevated above the surface of the water in order to be visible and then it was placed in different positions. The animals were requested to swim in water at four different directions of the tank in four trials. The obtained results were evaluated and extrapolated by the visio-motor coordination and motivational system.
2.6. Statistical Analysis
The statistical evaluations were carried out using the SPSS software version 23.0 The data were analyzed using one-way analysis of variance (One-way ANOVA) and repeated measures (RM) two-way analysis of variance (Two-way ANOVA) followed by Tukey’s post hoc test. The difference between the groups was statistically significant when the p-value was less than 0.05
2.7. Study Design
In this study, twenty-four male rats were divided into three groups. The first group was designated as the control group that received no treatment but was trained every day. The second group was coined as the sham group that received DMSO as a drug solvent. The third group (treatment group) received testosterone at a dose of 80 μg/0.5 μL DMSO/each side, dissolved in 0.5 μL vehicle (DMSO). Both of the sham and treatment groups were daily microinjected 30 min before the hidden platform test. The purpose of the experiment was to determine the impact of bilateral injection of testosterone into the CA1 region on the spatial learning and memory outcomes.
3. Results
3.1. Microinjection of Testosterone Into the CA1 Region Induced Spatial Learning Impairment
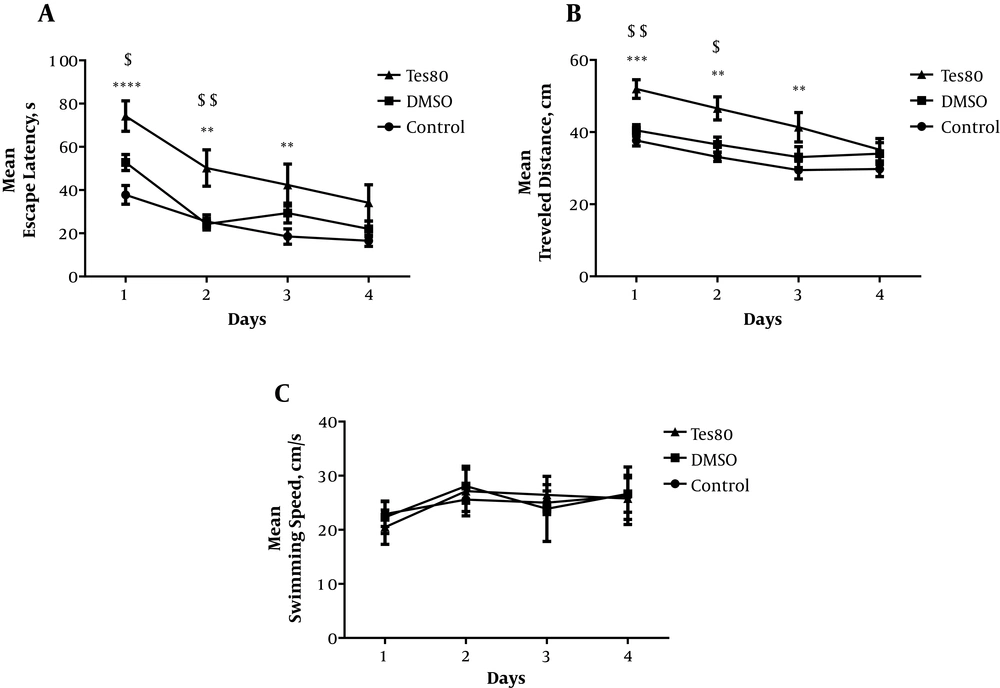
Figure 1 shows the results obtained from the injection of testosterone. The results indicated a significant effect of treatment [F (2.80) = 23.04; P < 0.0001] on the morphological changes in the CA1 region of the brain. There was also a significant increase in the escape latency in the first (P < 0.001), second, and third (P < 0.01) training days in the treatment group compared with the control group. Also, there was a significant increase in the escape latency in the first (P < 0.05) and second (P < 0.01) days in the treatment group when compared with the sham group (Figure 1A). Also, the findings showed a significant impact of testosterone administration [F (2, 80) = 19.55; P < 0.001] on travel distance parameters. The statistical analysis demonstrated a significant increment in the traveled distance in the first (P < 0.001), second, and third (P < 0.01) training days in the testosterone-treated group in comparison to the control group. Similarly, there was a significant increase in traveled distance in the first (P < 0.01) and second (P < 0.05) training days in the treatment group compared with the sham group (Figure 1B). There was no significant difference between the testosterone-treated group and other groups when the swimming speed was compared in all training days (Figure 1C).
The effect of testosterone on spatial learning. A significant increase was shown in the escape latency in training days in the treatment group compared with the control and sham groups (A). The statistical analysis demonstrated a significant increment in the traveled distance in training days in the testosterone-treated group in comparison to the control and sham groups (B). No significant difference was observed in the swimming speed among the groups (C).
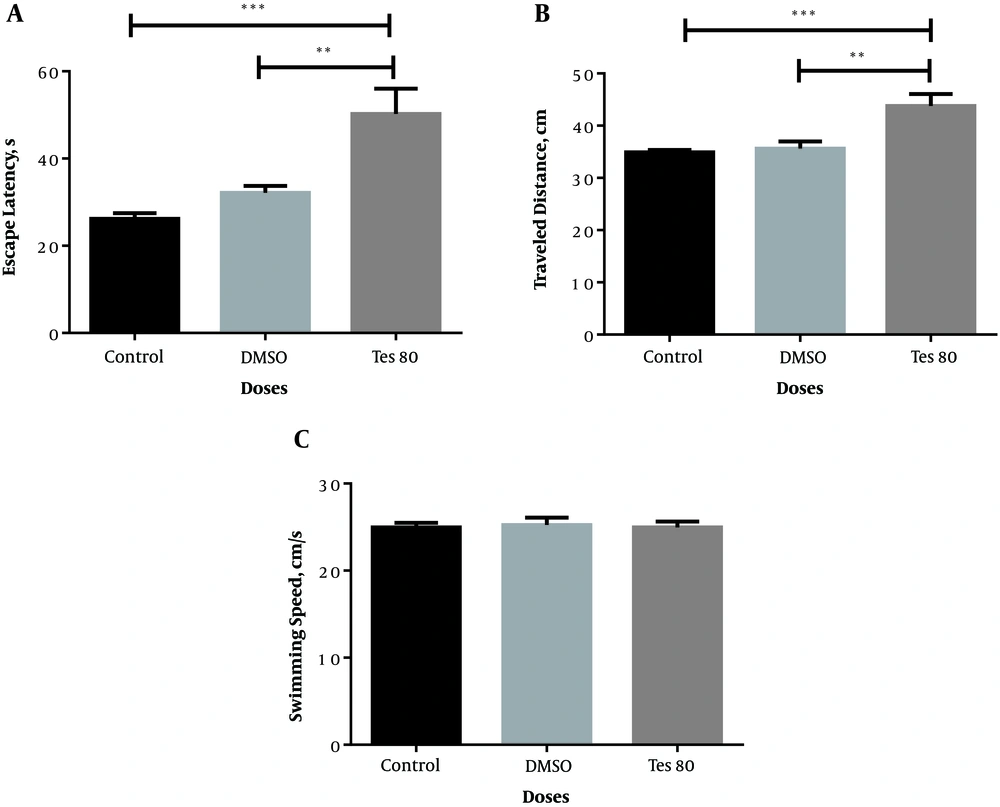
Figure 2 shows the comparative effects of testosterone injection in all experimental groups. A significant increase was found in the escape latency [F (2,20) = 13.942; P = 0.000] and traveled distance [F (2,20) = 10.247; P = 0.001] among the experimental groups (Figure 2A and B). No significant difference was observed among the studied groups considering the swimming speed (Figure 2C).
Average escape latency (A), traveled distance (B), and swimming speed (C) across all training days in the testosterone-treated group. Here, ** P < 0.01, *** P < 0.001 show significant differences among the experimental groups (A and B). No significant difference was observed among the studied groups considering the swimming speed (C).
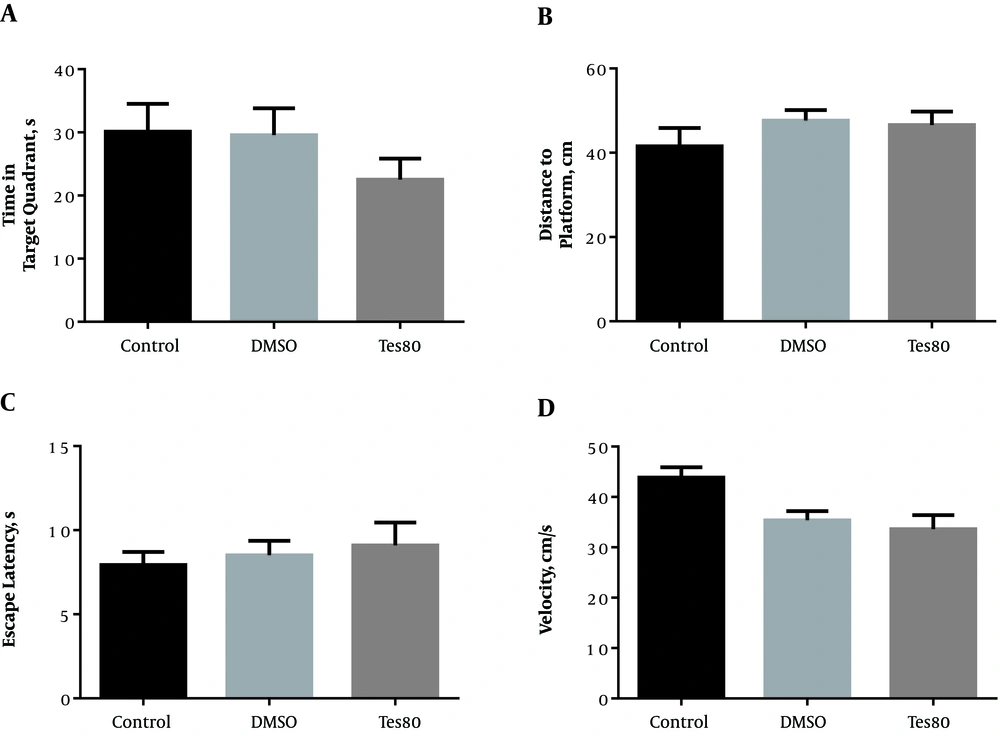
According to Figure 3, there was no significant difference between the treatment group and other groups regarding the time spent in the target quadrant (Figure 3A). Accordingly, there was no significant difference among all groups in terms of the traveled distance to the platform (Figure 3B). Of note, there was no significant difference among all groups of the study considering the escape latency (Figure 3C) or velocity (Figure 3D) in the visible platform test.
The effect of testosterone on probe test and visible platform test. No significant difference was shown between the treatment group and other groups regarding the time spent in the target quadrant (A) and the distance to the platform (B). There was no significant difference among all groups of the study considering the escape latency (C) or velocity (D) in the visible platform test.
4. Discussion
In the present study, we found that microinjection of testosterone (80 µg/0.5 μL DMSO/each side) into the CA1 region resulted in impairment in spatial learning but not in spatial memory and this may contribute to the memory formation. The behavioral tests were performed in all experimental groups as these examinations are considered the proxy of the retrieval stage in the memory formation. It is thought that the retrieval process is influenced by the memory trace intensity as the intensity of experience during the acquisition process in a training session could affect the retrieval memory (1). Thus retrieval stage during the formation of the memory is primarily defined as memory processes, which are requisite for the employment of information already in passive storage, and the retrieval stage is the only part of the memory creation that could be tracked by behavioral studies (14). Hence, a probe trial was utilized for the assessment of the reference memory at the end of learning (15). The probe trial is employed to verify the rats’ understanding of the location of the platform and observe the strategy that each rat follows when it discovers the platform is not there (16). We also used the probe trials for the evaluation of the rats’ ability to retrieve long-term memory and information learned in the hidden platform test. There are several possible explanations for the findings obtained in our study as mentioned below:
First, testosterone acts as a positive modulator for allosteric function of GABA (17) and also causes the activation of the GABAA receptor through binding to a particular site present in the interface between β and α subunits (18). Therefore, in our study, testosterone could lead to an increase in the concentration of GABA, as well as impairment in spatial learning.
Second, it has been alleged that memory fixation is empowered by the repetition of particular experience or tasks (19). Thus increasing the frequency of learning repetitions is capable of enhancing the memory fixation. In line with this, an increase in the spacing interval, i.e., the time period between learning repetitions improves long-term memory performance as well (20). Also, in animals that repeatedly encounter a familiar environment, there would be day-to-day turnover in CA1 pyramidal cells representing this environment, which enables the rats to distinguish the representations of different visits (21). It has been shown that behavioral experience alters the neuronal activity, inducing changes in the density of synapses and dendritic spine (22). Some studies have indicated the increased spine density in CAl pyramidal cells and basal dendrites in spatially trained rats. Concerning the unchanged length of the dendrites, a higher spine density reflects an expanded number of excitatory synapses per neuron, correlated with spatial learning, as well as altered connectivity. Furthermore, the trained animals showed increased learning ability as shown by faster acquisition in a water maze task. Studies demonstrated that behavioral training could cause a structural change in the hippocampal cortex of rats (23). Dendritic spines play a significant role in the interplay between experience and memory (24). The blockade of synaptic transmission in mature hippocampal slices leads to homeostatic spinogenesis that considerably elevates the number of stubby spines and non-synaptic filopodia, signifying a recapitulation of the early development (25). Therefore, regarding our results, the blockade of synaptic transmission by testosterone might increase the rate of spinogenesis.
Third, it has been demonstrated that LTP is comprised of two-time phases in comparison to memory. The short-lasting LTP triggers the long-lasting LTP when multiple trains of high-frequency stimulations are applied (26). Upon the induction of LTP, the number and activity of AMPARs, mediating synaptic transmission, as well as the number of spines would be increased (27). There is a correlation between LTP and the physical enlargement of spines, forming new spines from the neuronal shaft. Hence, the induction of LTP could result in an increase in the density of spines in neurons (28). Therefore, LTP is capable of causing structural changes in synapses (6). After the trigger of LTP, all of the local dendrites, nuclear transcription, and somatic translation incorporate to synthesize proteins required for the maintenance of the functional and structural plasticity. Actually, the occurrence of the LTP expression and maintenance does not sequentially happen; rather the structural changes such as the increase of spine growth and post-synaptic density would be evident after the induction. Though the early-mentioned structural alterations in spines have been studied in some details, the presence of LTPS may not be necessary as the synapses of the dendrites are capable of expressing and maintaining LTP (29). Studies indicate that LTP is an essential mediator of the induction process in the postsynaptic influx of calcium ions through the activated receptor of NMDA. Since the excitatory synapses of hippocampal pyramidal cells are mainly located on dendritic spines, calcium ions are presumably concentrated on the spine heads (29). In fact, spines could be considered a principle compartment for calcium ion (30).
The last reason is that testosterone is vital for the maintaining of the normal spine synapse density in the CA1 region of the male rats. Testosterone has a considerable impact on the spine synapse density in the CA1; however, this effect is primarily mediated via androgens instead of the estrogen receptors (13). The depletion of testosterone through castration cause a significant reduction in dendritic synapses and the administration of testosterone to castrated rats can retrieve the synaptic density to the baseline levels (31). According to our study, it is hypothesized that testosterone is able to increase dendritic spines and spatial memory.
4.1. Conclusions
Although testosterone causes the perturbations in learning, an increase in the frequency of spatial learning in MWM could lead to the repeated LTP, spinogenesis, increased spine density, and spontaneous generation of new spines, resulting in the improvement in spatial memory. On the other hand, the effect of testosterone through the androgen receptors causes an increase in dendritic spines leading to improved spatial memory in retrieval test.