1. Background
Green tiger prawn belongs to the Penaeus genus and Penaeidea family and is one of the first and indigenous and industrial shrimps in Iran, which exists in abundance, particularly in the warm season across the Persian Gulf (1, 2). Due to high water activity in the 0.95-one range, shrimp is food that spoils fast, and the International Committee on Food Microbiology has expressed the acceptable total count of live and durable psychrophilic aerobic bacteria in fresh marines as 106 CFU/g (3). Microbial growth is a major concern due to some microorganisms such as S. aureus and E. coli, which can potentially cause food-borne illness. It is proved that herbal essential oils and extracts enjoy antioxidant, anti-fungi, and antibacterial properties and also prevents unwanted interactions in food products (4, 5). Moreover, essential oils have organoleptic effects and create smell and taste in the products, which are unpleasant for the consumers.
One of the ways to minimize these unpleasant effects is using their nano-emulsions (6). Oil in water nano-emulsions in the 200 - 600 particle range have antimicrobial property, meaning that they mix with the bacteria cell wall or virus cover selectively and without any effects on the eukaryotes existing in the tissue and start the destruction of pathogens (7). However, this non-specialized mechanism does not lead to resistance in the strains either. Polylophium involucratum is of the Umbelliferae type, which is in Umbellifers order and mostly grown in temperate regions of the northern hemisphere. This plant has a pungent and penetrant smell. It is a local of Iran, and its habitat is in Mazandaran and the north heights of Alborz, Ramsar city (8, 9). It is used as a spice, flavor, and softener for meat in the north of Iran. This plant is used as a flavor in the Mazandaran province, yet despite the usage in foods, there have not been many efforts for separating its secondary metabolites (8).
2. Objectives
As per our knowledge, there is no report on the preservation of Green Tiger Pawn with nano-emulsion based on herb essential oils. In the present research, Polylophium involucratum essential oil nano-emulsion was prepared through phase return. Afterward, in order to study its effective role in preventing the quality decrease of a shrimp during storage, it was applied as a coating on the product. Then, the physicochemical, sensual, and microbial properties were assessed in order to evaluate the quality during storage.
3. Methods
3.1. Materials
Polylophium involucratum aerial organ was collected from Javaherdeh. Green Tiger Prawn was bought from among healthy and roughly the same size shrimps. All culture media (Muller Hinton Agar, Muller Hinton broth), and Tween 80 and Span 80 were supplied from Merck.Co (Germany). E. coli (ATCC 25922) and S. aureus (ATCC 29213) were also supplied from the Scientific and Industrial Organization Iran.
3.2. Essential Oil Extraction
A total of 150 gr of the ground plant along with distilled water were extracted with a Clevenger apparatus (Clevenger 1928) (10).
3.3. Essential Oil Constituents
The chemical constituents of plant essential oil were analyzed by gas chromatography-mass spectrometry (GC-MS, HP-6840/5973) based on the Saei-Dehkordi et al. (11) method.
3.4. Nano-Emulsion
Nano-emulsions were synthesized with phase return using the Duarte et al. (12) method. For this research, 1%, 2%, and 10% nano-emulsions were used. Firstly, four grams of essential oil, which is solved in oil, was mixed with surfactant mixture. Then, a total of 32 gr of distilled water was added to it as drops. Nano-emulsions containing 1% and 2% of essential oil were made the same way.
3.5. Particles Size
In this study, measuring nano-emulsion particles size was done in aqueous media using the dynamic light scattering (DLS) instrument (Marlon Company in Britain) (12).
3.6. Nano-Emulsion Stability
Long-term stability of storing the nano-emulsion produced assessed by measuring the changes in particles size and changes in their appearance (12).
3.7. Physical Stability
A total of 15 mL of nano-emulsion was poured into a laboratory tube with a cap and was placed in room temperature in a constant condition. After 28 days of storage, the supernatant oil layer was measured (13).
3.8. Bacterial Suspension
Bacterial strains were grown on Nutrient Agar at 37°C. A total of two to three colonies of the overnight culture of each strain were suspended in sterile physiological serum, and their opacity was adjusted with 0.5 MacFarland Turbidity Standard (14).
3.9. Antibacterial Properties
A total of 100 µg/mL of bacterial suspension (106 CFU/mL) was cultured in Mueller Hinton Agar, and the six mm paper disk was placed in specified intervals, then, 20 µL of pure, 10% essential oil, and 10% nano-emulsion were added to it. Then, after incubation at 37°C for 24 hours, the diameter of the zone made was measured (14).
3.10. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
In order to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of essential oil and nano-emulsion, broth microdilution method was employed using a 96 microwell plate, according to the standard method (15).
3.11. Tests on Fresh Shrimp Fillets
The first group of fillets was soaked in a container of 8.5 gr per liter physiological serum for 15 minutes. The second and third group of fillets were soaked in the 2% and 10% nano-emulsion and drained after two minutes, respectively. Sampling the packages was performed in four stages and done once every four days.
3.12. Weight Loss
The samples weight loss percentage in shrimp samples was calculated using the Duan et al. (16) method.
3.13. Total Viable Count and Psychrotrophic Total Count
Initially, one gr of the fillet was cut out in septic conditions and homogenized with nine ml of distilled water, and consecutive dilations of 10-1 to 10-8 were prepared and cultured through the superficial culture method (17).
3.14. pH Test
The pH level was measured by placing the electrode in the filtered liquid (18).
3.15. Color Test
The spectrophotometer chromatometer apparatus was employed using the method presented by Hunterlab Company, periodically on days zero, four, eight, and 12 from the coating time (19).
3.16. Statistical Analysis
Data analysis was conducted using SPSS software and ANOVA procedure at the 0.05 level of significance. The related means were analyzed with the Duncan test at the 0.05 level.
4. Results
4.1. Essential Oil Analysis
The density of essential oil was calculated as 0.8 gr in ml. The main constituents of essential oil, gamma terpinene (16.08%), cuminaldehyde (14.45%), gamma therpinen-7-ol (13.03%), and p-cymene (12.19%). The efficiency of the essential oil was determined as 1.74%.
4.2. DLS
The results of DLS exists a peak, which was indicative of the mean size of about 68.3 nanometers.
4.3. Nano-Emulsion Sustainability
In order to assess the sustainability of nano-vectors, some technics can be employed. The average of particles sizes reached 136.2 nanometers.
4.4. Creaming Test
After 28 days of storage, no oil layer was formed on the 10% nano-emulsion; hence, the stability of the synthesized nano-emulsion is confirmed via this test. The 1% and 2% nano-emulsions were sediment and turbid after 48 hours and 13 days, respectively.
4.5. Antibacterial Properties
The average diameter of growth inhibition zone for nano-emulsions in diffusion in the agar method were 10.4 mm and nine mm for S. aureus and E. coli, respectively; the same values were obtained as 35 mm and 26 mm for pure essential oil. The average diameter of growth inhibition in diffusion in the vapor phase was obtained zero against the afore-mentioned bacteria for the nano-emulsion, and the same values were reported as 32 and 36 for the pure essential oil. The values obtained for the MIC and MBC of the pure essential oil against S. aureus were reported as 12500 and 2500 µg/L, and the same values for E. coli bacteria were reported as 6250 and 25000. The least growth inhibition concentration and least lethal concentration for the 10% nano-emulsion against S. aureus was obtained as 50000 and for E. coil, 25000 µg/L (Tables 1-3).
| Bacteria | Essential Oil Nano-Emulsion | Essential Oil | Gentamycin | Chloramphenicol | |||
|---|---|---|---|---|---|---|---|
| 10% | 2% | 1% | Pure | 10% | 10 µg | 30 µg | |
| E. coli | 9 ± 0.12 | - | - | 26 ± 0.32 | 8.8 ± 0.41 | 38 ± 0.24 | 42 ± 0.11 |
| S. aureus | 10.4 ± 0.27 | - | - | 35 ± 0.18 | 1020 ± 0.28 | 52 ± 0.12 | 34 ± 0.25 |
The Average Diameter (mm) of the Zone Formed via Diffusion in Agar Method
| Bacteria | Essential Oil Nano-Emulsion | Essential Oil | Gentamicin | Chloramphenicol | |||
|---|---|---|---|---|---|---|---|
| 10% | 2% | 1% | Pure | 10% | 10 µg | 30 µg | |
| E. coli | Zero | Zero | Zero | 36 ± 0.23 | 10 ± 014 | Zero | Zero |
| S. aureus | Zero | Zero | Zero | 32 ± 0.24 | 15 ± 0.21 | Zero | Zero |
The Average Diameter (mm) of the Zone Formed via Vapor Phase Diffusion Method
| Bacteria | Pure Essential Oil, µg/L | Nano-Emulsion, µg/L | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| E .coli | 6250 ± 0.33 | 25000 ± 0.38 | 25000 ± 0.15 | 25000 ± 0.25 |
| S. aureus | 12500 ± 0.23 | 50000 ± 0.25 | 50000 ± 0.15 | 50000 ± 0.35 |
MIC and MBC
4.6. Weight Loss Changes
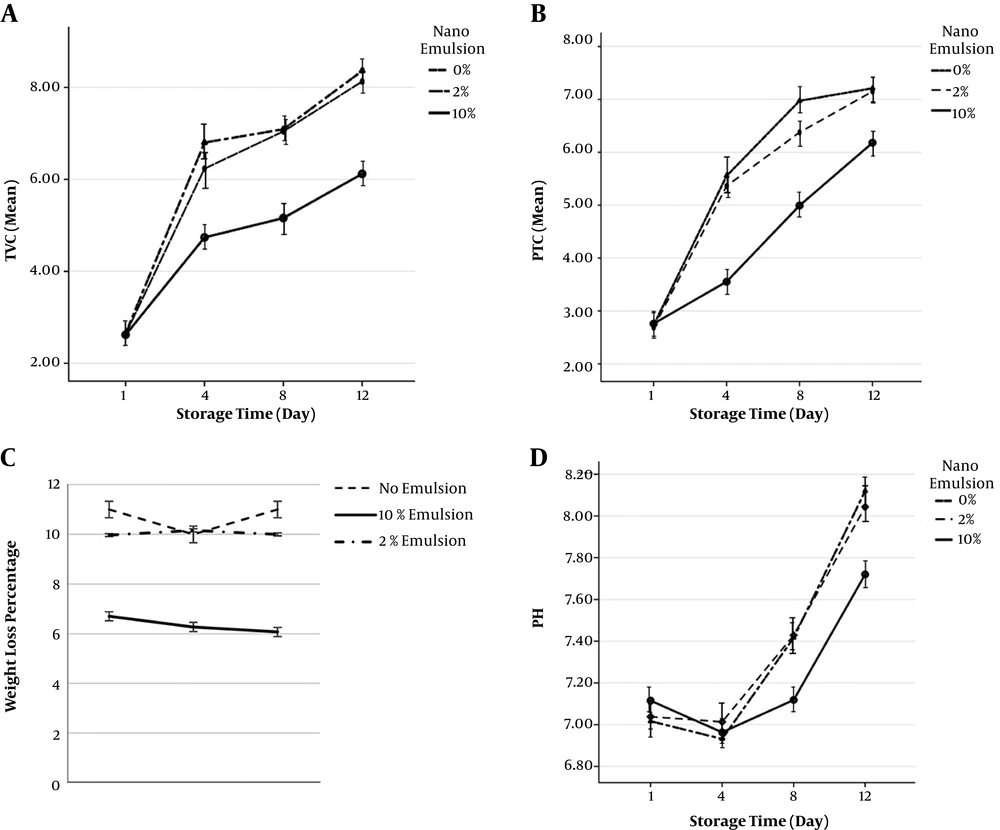
The changes in weight loss in the coated shrimp samples in comparison with control samples are shown in Figure 1. The results show that the highest weight loss level (11%) is in the control sample and the lowest level (6.07%) belongs to 10% nano-emulsion, and as a result, the 10% concentration of nano-emulsion had the most control over the weight loss percentage of shrimp fillet under study (α = 0.05).
4.7. Total Viable Count and Psychrotrophic Total Count
The changes in the number of mesophilic bacteria in different shrimp samples during the 12 days of storage are shown in Figure 1. On the first day of storage, total viable count (TVC) was between 2.60 to 2.65 (Log CFU/g) in all of the samples under study, and there was a significant statistical difference. However, with time, the total count of bacteria significantly increased in all of the samples. Nevertheless, TVC in the tissue coated with 10% nano-emulsion had a much moderate incremental slope than the other treatments. The highest TVC belongs to the statistical mean of the control sample on day 12. The results obtained in this study showed that psychrotrophic total count (PTC) significantly increased in all of the samples during the storage period. The highest mean of PTC measured in shrimp fillet tissue belonged to the statistical mean of control sample on day 12. The number of these bacteria increased from 2.74 to 7.20 (Log CFU/g) after 12 days of preservation in the fridge.
4.8. pH
These changes during 12 days are presented in Figure 1. Results showed that pH increases until the end of the storage period. On day one of the storage, the average pH of the control sample was 7.017, which reached 8.120 after 12 days, which was the highest increase among the samples.
4.9. Chromatography
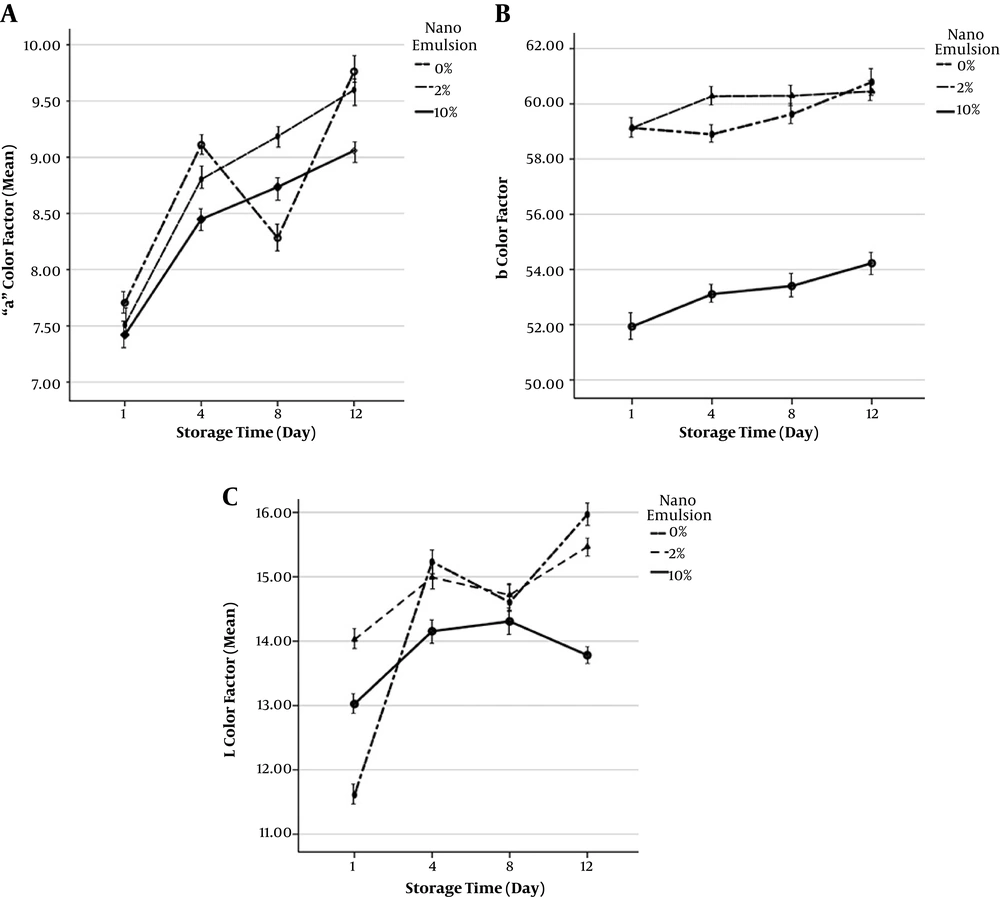
Factors in A chromatography demonstrate the red-green level of the samples. In the same order, the highest level of the A color factor measured in the shrimp tissue was related to the statistical mean of the sample devoid of the nano-emulsion solution on day 12. Figure 2 shows the fluctuations of L chromatographic factor of shrimp samples during the 12 days of storage in the fridge. The highest mean of the L color factor level measured in the same way in the shrimp fillet tissue belongs to the statistical mean of the sample devoid of the nano-emulsion solution on day 12. Furthermore, B chromatographic factor indicates the yellow-blue color of the samples. The least mean of the B color factor measured in the shrimp fillet tissue was related to the statistical mean of 10% nano-emulsion sample on day one (P > 0.05).
5. Discussion
The results of the present research indicate that the average size of the 10% nano-emulsion particles prepared via return phase method using the dynamic light scattering method was determined as 68.3 nanometers. Moreover, the results of particles size through DLS showed a slight difference in the size of nano-emulsion particles after 60 days, as the average size of particles reached 136.2 nanometers after this time. This increase can be due to the Ostwald Ripening phenomenon and the integration of drops due to storing the nano-emulsion in room temperature for a long time. O/W nano-emulsions are oil drops scattered in water with an unstable nature and in fact, the Gibbs energy is positive during the formation of free nano-emulsion (20).
The reason for the identical results in essential oil and nano-emulsion bactericide level against E. coli may be the large distance between the consecutive dilations of the essential oil or nano-emulsion. The reason for comparing nano-emulsion with 10% essential oil is the amount of essential oil used in producing the 10% nano-emulsion. In general, the effect of herbal essential oils and their mixtures are somehow more influential on positive gram bacteria than on the negative gram ones (21).
Weight loss in samples is one of the chief reasons for the marketability decline of fresh food products, which happens due to humidity decrease in the samples. The level of weight loss in samples coated with 10% essential oil nano-emulsion was less severe, and in the samples coated with 10% nano-emulsion, it was almost half of that of the control samples. It was also expected that due to the hydrophilic nature of the oil in water essential oil, the weight loss ratio in the coated samples would be higher; however, the results demonstrated that this nano-emulsion property does not influence the higher weight loss ratio in the coated samples. Scientists reported that coating salmon fish meat with chitosan leads to humidity preservation in the coated samples and in comparison with the control sample, it led to a decrease in weight loss from 17.8% in the control sample to 11.9% and 12.9% in the coated samples (22).
Different concentrations of nano-emulsion have never been effective in controlling the A, B, and L color factors (P > 0.05). On the other hand, the 10% nano-emulsion, regardless of the interference of the time factor, has been significantly more effective on the control of the B color factor level as a variable. The results of the other study demonstrated that all of the coated samples, in particular, the samples coated with oil nano-particles containing zenian essential oil together with Aloe vera, had higher amounts of A during the storage time, which refers to the better preservation of meat red color in these samples as compared with the control sample (23)
The results show that using 10% essential oil nano-emulsion lowers the pace of increase in the number of psychrophilic bacteria (24). In this research, the strong influence of 10% essential oil nano-emulsion in inhibiting the psychrophilic bacteria was observed in a way that this number was obtained according to the 6.17 Log cfu/g equivalence, which is an acceptable level for human consumption (25). The results of this research are in congruence with those of the other researchers (26).
5.1. Conclusions
It can be concluded that considering the increasing tendency toward using herbal and natural constituents as a substitution for chemical preservatives, the initiation of modern nano-materials and nano-essential oils science and technology in the field of increasing food products shelf-life, as well as the findings of this research, the antimicrobial property of 10% essential oil nano-emulsion on reducing the microbial population and increasing the preservation period of green tiger prawn in the fridge is confirmed.