1. Background
Polyvinyl chloride (PVC) is an important raw material in the chemical industry. It is used to make plastic containers, coated wires, and in the automotive industry. It can cause harmful effects on the central nervous system in humans (1-3). The PVC is used in most devices for humans and associated with the spread of diseases (4). Since PVC is not chemically bonded with polymer, it can be separated easily (2, 3). Therefore, it can enter the human body with eating food, drinking water, transmission through the air, or even contact with medications (1, 5) and toys (6, 7), especially in children. Another application of PVC is in the medical equipment and laboratory materials (1). Medical and surgical applications such as injection devices, blood storage bags, chip tubes, and dialysis machines contain plenty of PVCs. studies have shown that people who have used a large number of blood products, the PVC substance has been detected in their blood and tissues (8). The food is the main source of PVC transmission to humans (7).
In people who have exposed to severe PVC, liver cancer has been observed 15 times more than normal humans, and in this group of people, brain cancer has been 4 times higher than others (9). The PVC is one of the most abundant poisons in the natural environment of humans. This material is one of the factors that produce oxidative stress enzymes and also causes some enzymes to impair (10). Research results have shown that PVC contributes to some neurodegenerative diseases. Vitamin E is one of the most important antioxidants that can prevent damaging effects leading to a number of diseases, such as diabetes (11-13), stroke (14, 15), Alzheimer (16, 17), and aging (18).
2. Objectives
In this study, the effect of vitamin E was investigated against PVC in rat’s oxidative stress enzymes.
3. Methods
We used 24 male Wistar rats (weighing 180 - 200g) in the following groups, the control (N = 6), Vit E (N = 6), PVC (N = 6), PVC plus Vit E (N = 6) groups. The animals hosted in standard condition. We orally administered vitamin E (150 mg/kg.bw/day) and PVC (1000 mg/kg.bw/day) for 40 days. The PVC (Merck, Germany) and vitamin E were used as described in previous studies (11, 12).
3.1. Lipid Peroxidation Assay
Rats were sacrificed after 40 days of exposure to PVC. The right hemisphere was removed, homogenized, and the level of malondialdehyde (MDA), catalase (CAT), and total antioxidant capacity (TAOC) were evaluated.
3.1.1. Measuring Brain Malondialdehyde Level
To this end, 0.2 g of the brain tissue was transferred to phosphate buffer saline (PBS) 0ºC, 0.05 M, pH = 7.4 (10% w/v), and homogenously ground by a mortar and pestle. Then the solution was centrifuged at 1000 rpm. Afterward, 150 μg of the supernatant of the centrifuged specimen was removed, 300 μg of 10% trichloroacetic acid was added, and it was centrifuged at 1000 rpm, 4ºC, for 10 min. Then 300 μL of the supernatant was transferred to the tube and incubated with 300 μL of 0.67% thiobarbituric acid at 100ºC for 25 min. After 5 minutes of cooling the solution, the color pink resulting from the reaction between MDA and thiobarbituric acid appeared and was evaluated with a spectrophotometer at 535 nm wavelength. The concentration of MDA was calculated using the MDA absorption coefficient in nmol/g tissue (13).
3.1.2. Catalase Activity Evaluation
Catalase activity was determined based on its ability to decompose H2O2 using the Aebi method. In this regard, H2O2 decomposition can be assessed by absorption reduction at 240 nm. The difference in absorption per unit of time equals catalase activity. First, 10% w/v of 0.2 g of brain tissue was washed with ice-cold PBS (pH = 6.8) and homogenously ground by a mortar and pestle. The homogenized tissue solution was centrifuged at 5000 rpm for 5 min. Next, 100 μL of the centrifuged supernatant was added to 2.8 μL of PBS. Then 100 μL of H2O2 solution was added, and the absorption was measured in the 240 nm wavelength at 0 and 30 s. The PBS was used as the blank. In the end, values were expressed as U/g tissue (14).
3.1.3. Total Antioxidant Capacity Measurement
The TAOC of brain tissue was measured using the ferric antioxidant power test (FRAP). On the day of the evaluation, tissues were homogenized in cold KCL solution. To prepare homogeneous tissue solution, 0.2 g of brain tissue was taken, 10% (w/v) of it was added to KCL solution and homogenously ground by a mortar and pestle. Then the prepared homogeneous solution was centrifuged at 1000 rpm for 5 min. Afterward, 100 μL of the supernatant was removed and transferred to a test tube, 3 μL of FRAP indicator was added, and it was incubated at 37ºC heated-bath for 7 - 10 min. The absorption of the blue complex was read using a spectrophotometer at 593 nm. Values were expressed as mmol/g tissue (15).
3.2. Statistical Analysis
Data of the groups were analyzed by SPSS software and ANOVA and Tukey’s tests were used. The significance level was considered P < 0.05.
4. Results
4.1. Biochemical Test Results
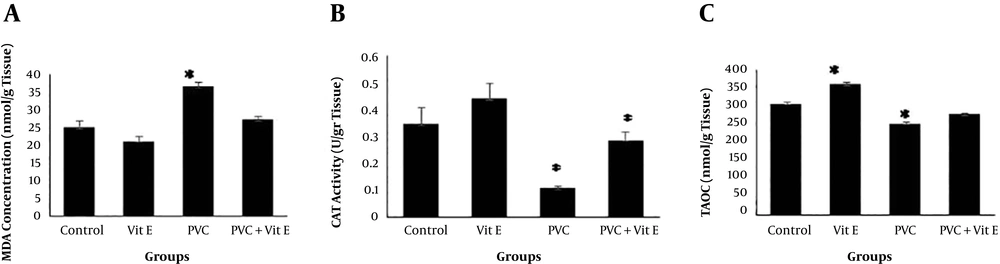
MDA concentration was increased in the PVC group, notably compared to the control and the vitamin E groups (P < 0.05). However, MDA concentrations in the PVC + Vitamin E group were similar compared to the vitamin E and control groups (P > 0.05) (Figure 1A).
The CAT activity demonstrated a significant reduction in groups under the treatment with PVC versus the control group (P < 0.05). Nevertheless, this increase was not significant in the vitamin E group compared to the control group (P > 0.05). No significant difference was seen between groups receiving PVC plus vitamin E and the control group (P > 0.05) (Figure 1B).
For TAOC, a considerable reduction was found in the group under the treatment with PVC compared with the control group (P < 0.05). However, no discrepancy was observed in PVC plus Vit E group (P > 0.05). Furthermore, TAOC was significantly higher in rats treated with vitamin E in comparison to the control group (P < 0.05) (Figure 1C).
5. Discussion
The present study showed that male rats exposed to PVC displayed a significant decrease in the antioxidant properties of the brain. The scientists have explained, PVC or PVC metabolite exposure could create significant neuronal degeneration and decrease the membrane’s permeability (8). Thus it seems PVC can decrease neural plasticity.
The PVC exposure may increase pathological changes in receptor activity and create toxic effects via the production of reactive oxygen species (ROS). On the other hand, PVC can reinforce the toxic effects of ROS via the reduction of antioxidant enzymes or radical scavengers (10). In the present study, PVC treatment significantly increased malondialdehyde and decreased catalase activity and total antioxidant capacity in the brain. These results confirmed that PVC is accompanied by an increased generation of ROS products (16, 17). Oxidation of the sulfhydryl is ensured from a combination of superoxide and hydrogen peroxide, which disturb glutathione synthesis via competitive antagonism of cysteine transport and provoke the genesis of free radicals through thiol oxidation (18).
The studies explained that oxidative stress had an important role in PVC-induced brain changes (19). Also, it has been shown that neurons can defend themselves and decrease excitotoxic effects of oxidative agents (18). The scientists have been interested in evaluating the antioxidant defense mechanisms of the brain (18, 20-26). On the other hand, it is evident that glial cells produce the neurotrophic factors and cytokines that decreases-induced neurotoxicity. Astrocytes have more antioxidant strength than neurons and defend neurons against oxidation and support for neuronal life (27). Induction of oxidative stress after PVC exposure and progressive increment of ROS production result in neuronal death via protein oxidation, DNA damages, and increased amount of lipid peroxidation in neural membrane (28). It has been shown that the brain produces slow endogenous levels of vitamin E (an effective antioxidant agent), and it is mainly an easy target of oxidative stress (29).
The main finding of the current research is the effects of vitamin E on oxidative stress on the brain after exposure to PVC. In recent years, it has been shown that vitamin E protects some organs against oxidative stress (30). Also, it has been explained that in the developing cerebellum, vitamin E administration reduced alcohol-induced oxidative stress and apoptosis (18). The studies established that vitamin E has similarly valuable effects on glial cells versus toxic effects of the chemical and/or metabolic agents. It can protect nerve tissues by the clearance of the free radicals and stabilizing the cell membranes (27). These data show that the administration of vitamin E may reduce lipid peroxidation and keep the neurons from PVC-related oxidative stress.