1. Background
The shortened sleep duration in modern life has produced some health problems although there is growing awareness that healthy sleep is important to improve the quality of life in all societies. Sleep research is a relatively new scientific field in the clinical management of sleep disorders to reduce their effects (1). Recent studies have examined the effects of sleep restriction on human and animal models. Human studies have shown that daily sleep of less than 6 hours is associated with mortality, including cancer-related mortality, and morbidities such as type II diabetes, cardiovascular diseases, and obesity (2-4). These findings are consistent with the studies on animals (5). Sleep restriction (SR) is related to a range of nervous and psychological diseases, such as depression and epilepsy (6, 7). Depression is defined as a stress-related disease (8). There are some proofs that SR-induced stress and depression can have destructive physiological effects, probably resulting in death in animals (9, 10).
Sleep is divided into two stages of rapid eye movement (REM) and non-REM (NREM) sleep. Sleep deprivation induced by the columns-in-water (modified multiple platforms) method has been used to restrict the non-REM and REM sleep, but depending on the diameter of the platform, it mainly eliminates REM sleep. Therefore, the animal would be mainly deprived of REM sleep depending on the diameter of the platform. Rats fall into the water and awaken due to the decrease in muscle tone in REM sleep (11).
In recent years, some researchers reported significant relationships between psychiatric disorders of sleep deprivation and different hormones (12). It seems that norepinephrine (NE) in the locus coeruleus is the most important agent to induce behavioral depression (13). The production of NE by tyrosine hydroxylase and dopamine-β-hydroxylase is probably a mechanism to understand behavioral depression (14). On the other hand, some studies showed that sleep deprivation for 2 or 3 weeks in rats could reduce thyroxine (T4) in serum and increase thyrotrophin-releasing hormone (TRH) mRNA expression, whereas serum thyroid-stimulating hormone (TSH) was not affected (15). Balzano et al. observed the reduction of plasma T4 concentrations and the significant increase of iodothyronine deiodinase type II activity in the brown adipose tissue of rats (16). Also, Bergmann reported the decreased plasma concentrations of thyroxine and triiodothyronine (T3) in rats during sleep deprivation (17).
Regular exercise has a positive influence on health and sleep quality. For example, some studies showed that exercise could have antidepressant effects and made protection against the harmful consequences of stress and depression (18, 19). The forced swim test (FST) was developed by Porsolt for putative antidepressant activity in rodent model (20). In the basic protocol of FST, the animal is placed in a container filled with water, from which the rat cannot escape; then, the time of immobility posture is recorded. The duration of immobility and the latency to the beginningof immobility are the main dependence criteria (21, 22). The FST can assess the depressive-like manners in animals to study the psychological diseases (23, 24).
Although sleep deprivation has been well studied, developing knowledge about psychiatric disorders induced by SR and assessing the impact of exercise on them still need further study.
2. Objectives
The purpose of the current study was to examine the influence of exercise and SR on depression assessed by the FST in male rats. In addition, the concentrations of T4 and NE hormones were assessed.
3. Methods
3.1. Animal
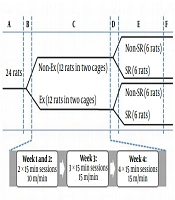
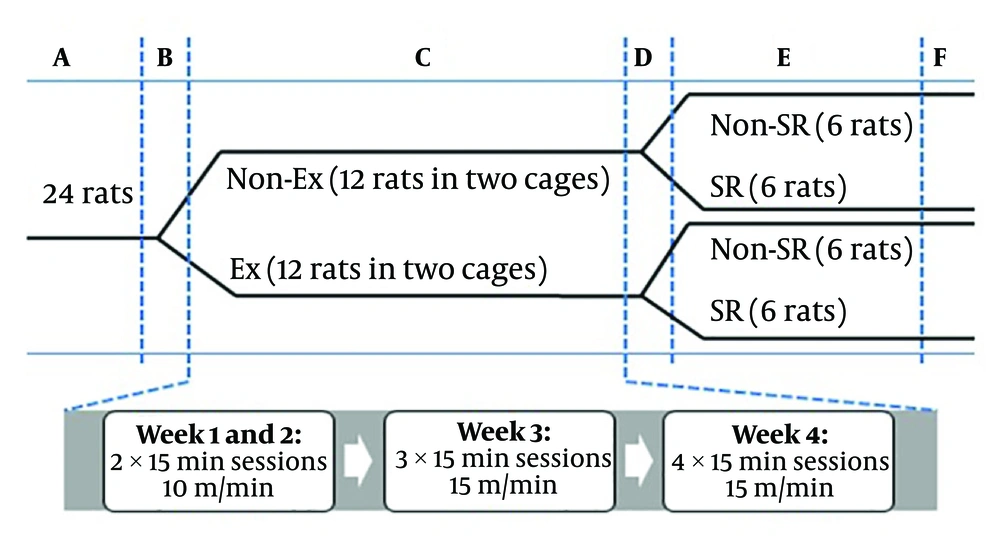
Male Wistar rats were included in this study and housed in a chamber with a 12-h light/dark cycle at 24 ± 1°C temperature and 55% - 60% humidity. All rats had two months of age weighing 226.04 ± 15.09 g at the beginning of the experiments. They had access to food and water ad libitum and acclimatized for at least one week before any treatment. Rats were randomly housed in four groups (6 rats per cage) including without exercise without SR (Non-Ex-Non-SR), without exercise with SR (Non-Ex-SR), with exercise without SR (Ex-Non-SR), and with exercise with SR (Ex-SR). We tried to reduce the total of animals used and their distress. The experimental schedule is shown in Figure 1.
The experimental schedule. A, acclimatization period (about 7 days); B, pre-exercise period (2 days); C, exercise period (4 weeks); D, resting period (1 day); E, sleep restriction period (7 days), and F, FST, blood sampling and hormone assessment. Ex and Non-Ex, with and without exercise; SR and Non-SR, with and without sleep restriction, respectively.
3.2. Treadmill Exercise
The animals in the exercise groups ran for five days per week (from Saturday to Wednesday) during a period of brightness (10:00 to 16:00) for four weeks. Before the beginning of the test, the animals were adapted to the treadmill at 30 min/day for two days (pre-exercise period) to decrease the stress induced by new condition. According to the method adopted from Zagaar, at the beginning of the test, rats ran with a speed of 10 m/min for 15 minutes in two rounds for two weeks (25). In the third week, the speed was raised to 15 m/min for 15 minutes in three rounds while in the fourth week, it was for 15 minutes in four rounds. Between each round, the rats had a 5- minutes rest period. We observed the rats throughout the test to confirm their constant running during the rounds and to find out any signs of possible difficulties such as pain or exhaustion. In no exercise groups, the animals were placed in the setup in the same condition with the exercise groups (the same time and the same number of sessions) but without running and at the end of each test, the rats had access to water (25).
3.3. Induction of Sleep Restriction
We used the setup of SR induction (columns-in-water model) according to Alhaider et al.. Rats fell into the water through the rapid eye movement sleep to the decrease the muscle tone and awake (11). Rats in the SR groups were placed in an aquarium at the room temperature. The cage mates (6 rats) were put together in an aquarium because of social stability. We placed 15 columns in the aquarium and each platform had a diameter of 5 cm and was 2 cm above the water level. The columns were arranged in 3 rows with a distance of 7 cm (edge to edge) such that rats freely moved between platforms. The food pellets were freely available to the rats and clean water bottles placed on the top of the aquarium during the entire length of the experiment. In the present research, after one rest day, SR was induced for 16 hours per day during 5:00 pm to 9:00 am for one week. Sleep restriction was induced for 24 hours after execution of the last exercise in the exercise groups (26). All rats were weighed at the beginning and the end of the exercise protocol, as well as after SR.
3.4. Forced Swimming Test
The FST was used to assess the depressive-like behavior according to Porsolt’s method (20). Rats were dropped individually in a glass tube with 40 cm in height and 20 cm in diameter that contained 25 cm of water maintained at 25°C. After the first 2 minutes, the total duration of immobility, climbing, and swimming were assessed for 6 minutes in each test. An immobile rat was floated motionless or made only those movements that needed to keep its head out of water. The trying of the rats to climb the wall or jump out of the tank was considered as climbing. Also, an active behavior for movement around the surface of water was considered as swimming (27).
3.5. Blood Sampling and Hormone Assessment
Considering the pre-exercise period, the test was finished on the 38th day. Then, the rats were anesthetized by chloroform and immediately, blood samples were collected from their hearts. After coagulation, the serum was extracted by centrifugation (3,500 g for 15 minutes) and kept at -20°C. In the next step, NE and T4 were measured using Enzyme-Linked Immunosorbent Assay (ELISA) according to the kit manufacturer’s protocol.
3.6. Statistical Analysis
We analyzed our data using statistical analysis software (28). The Analysis of Variance (ANOVA) was used. P values of < 0.05 were considered statistically significant. Tukey test was used to compare differences between groups. The data were shown as mean ± SE.
4. Results
4.1. Weight Gain
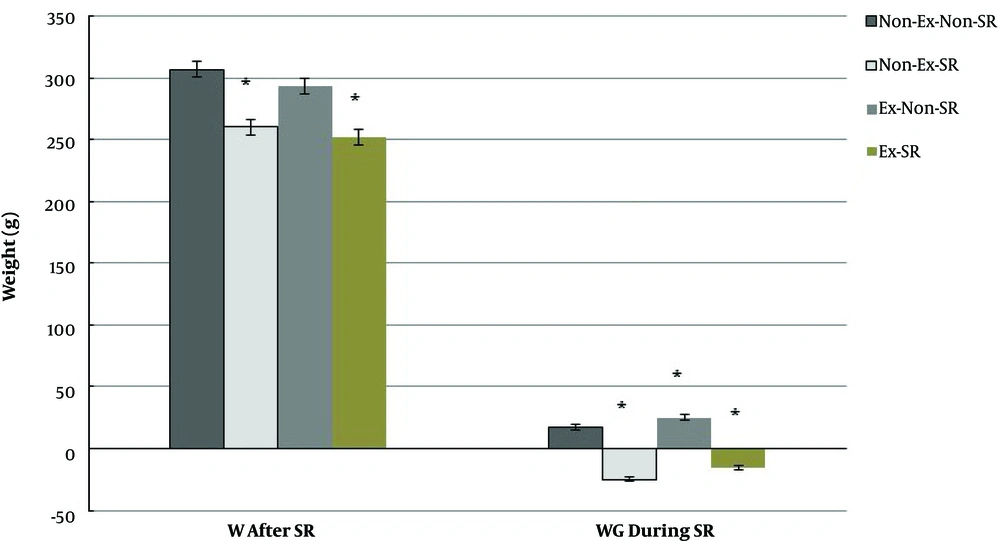
Figure 2 summarizes the bodyweight changes and weight gains in the whole procedure. After SR, the average body weight of rats was significantly lower in the Ex-SR group (252.00 ± 8.41 g) than in Non-Ex-Non-SR group (306.83 ± 22.95 g, P < 0.0001) and Ex-Non-SR group (293.50 ± 14.40 g, P = 0.001). However, we did not see any significant differences between the Ex-SR and Non-Ex-SR groups (P = 0.78) and between the Ex-Non-SR and Non-Ex-Non-SR groups (P = 0.44) (Figure 2).
Means and standard errors of weight (W) and weight gain (WG) of rats during sleep restriction (SR) period. Means are statistically different (P < 0.05). Non-Ex-Non-SR, without exercise without sleep restriction; Non-Ex-SR, without exercise with sleep restriction; Ex-Non-SR, with exercise without sleep restriction, and Ex-SR, with exercise with sleep restriction.
Sleep restricted rats in Non-Ex and Ex groups showed 24.83 ± 6.85 and 15.50 ± 4.42 g weight losses, respectively; in the Non-SR groups without and with Ex, weight losses were 17.00 ± 3.63 and 25.17 ± 4.54 g, respectively (Figure 2). The results showed significant differences between the mean weight gains of all groups (P < 0.05).
4.2. Forced Swimming Test
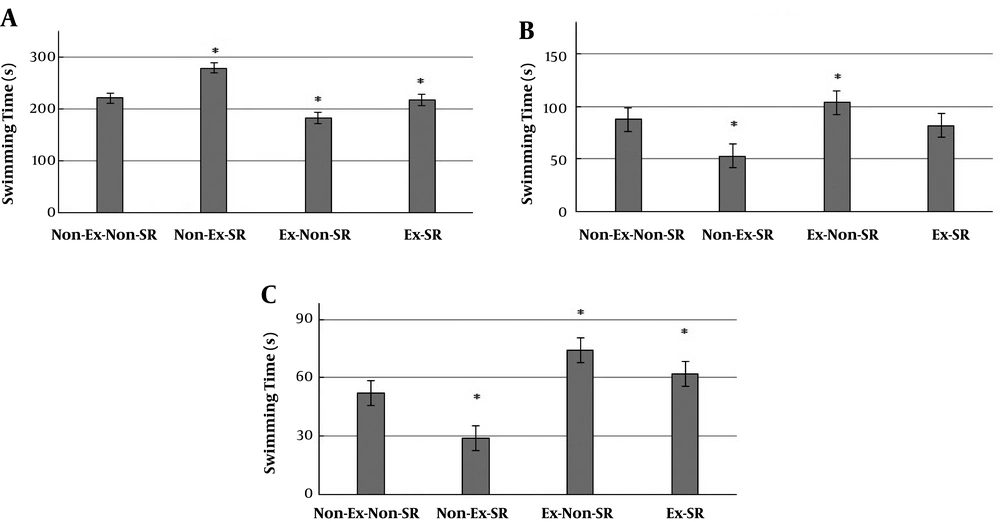
The results of the FST are shown in Figure 3. The mean duration of immobility in the Non-Ex-Non-SR group was the highest (220.50 ± 19.35 seconds) and that of the Ex-Non-SR group was the lowest (182.17±22.36 seconds). On the contrary, the climbing (74.00 ± 16.57 seconds, P = 0.023) and swimming (103.83 ± 38.95 seconds, P = 0.0003) durations in the Ex-Non-SR group were higher than those in the Non-Ex-SR group. In general, the highest difference was observed between the behavior of Non-Ex-SR and Ex-Non-SR groups.
Influence of exercise and SR on depressive-like behavior in rats. Means are statistically different (P < 0.05). Non-Ex-Non-SR, without exercise without sleep restriction; Non-Ex-SR, without exercise with sleep restriction; Ex-Non-SR, with exercise without sleep restriction, and Ex-SR, with exercise with sleep restriction.
4.3. Levels of Hormones
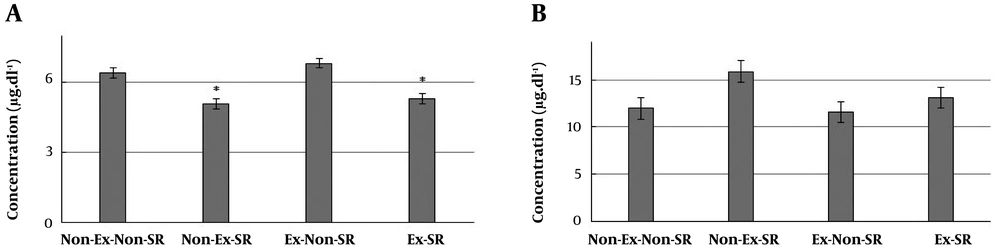
The means and standard errors of T4 and NE in different groups are shown in Figure 4. In sleep-restricted rats, the plasma concentrations of T4 hormone decreased significantly. The Ex-Non-SR group with the highest value of 6.81 ± 0.38 µg dL-1 had significant differences with Non-Ex-SR (5.08 ± 0.57 µg dL-1, P < 0.001) and Ex-SR (5.30 ± 0.55 µg dL-1, P < 0.001) groups. Also, Non-Ex-Non-SR group (6.41 ± 0.58 µg dL-1) had significant differences with Non-Ex-SR (P = 0.003) and Ex-SR (P = 0.012) groups (Figure 4A).
The groups of sleep-restricted rats showed high levels of NE. However, there were no significant differences between all the groups. In general, the lowest (11.60 ± 2.38 ng.mL-1) and the highest (15.88 ± 3.11 ng. mL-1) values were found in Ex-Non-SR and Non-Ex-SR groups, respectively (Figure 4B).
5. Discussion
weight reduction in our study is comparable to other studies conducted by Blanco-Centurion and Shiromani (29) and Mazzeo and Horvath (30). In the study by Blanco-Centurion and Shiromani, the exercise decreased significantly (P < 0.05) body weight at the end of the first week of exercise, and this effect was constant at the end of the eighth week (29). Mazzeo and Horvath reported that exercise decreased body fat (30).
The researchers also showed that REMs deprivation in animals created severe physical disorders, such as skin lesions and weight loss (17, 31). Rodrigues showed that sleep deprivation reduced body weight gain (32). Paradoxical sleep deprivation and sleep restriction can increase sympathetic activity, thereby increasing metabolic rates and decreasing body weight gain (33). The changes in T3 and T4 concentrations may also be the mechanism underlying body weight loss induced by sleep deprivation in rats. On the other hand, thyroid hormones and NE signaling pathways are interconnected and can affect each other (32).
Bodyweight loss was lower in the Ex-SR group than in the Non-Ex-SR group. It means that rats without exercise protocol were more susceptible to sleep restriction. It seems that rats in the Ex-SR group could gain experience and resistance.
On the other hand, exercise has several helpful effects on cognition and decreases some destructive effects of stress in humans and rodents (34). In rodents, the voluntary exercise reverses learning deficits induced by stress in the shuttle box and reduces helpless behavior measured by the FST (24, 35). Similar effects were found in human studies showing that exercise had antidepressant effects (36). The studies have shown the effect of exercise on the induction of protein and peptide release, which, in turn, improves the health and survival of neurons, such as insulin-like growth factor, brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor (8, 37).
In the FST, the duration of climbing behavior is considered a sign of the improvement of animal depression (8). In this study, the increased duration of immobility in the Non-Ex-SR group indicated a depressive-like condition in rats (8, 20, 23). Rats with given exercise treatment displayed significantly fewer stops than rats in the groups without exercise. Thus, the exercise made them more active and less depressed, as also observed by Malisch et al. (34), Dunn et al. (36), and Rygula et al. (24).
In a study by Bergmann et al., it was reported that the restriction of REM sleep in rats significantly decreased the level of T4 and T3 hormones (17). In another study, sleep restriction had no effect on TSH and T4 plasma levels (32). Rodrigues mentioned that REM sleep restriction both for 24 hours and for 96 hours could decrease serum TSH and T4 concentrations whereas it increased T3 as compared to control animals (P < 0.05) (32). Parekh et al. demonstrated that sleep deprivation could increase T3, T4, and TSH concentrations in humans (38). Various durations of sleep restriction in studies make it difficult to compare the results. However, studies on rats showed that the total sleep deprivation created a decrease in T3 and T4 concentrations and the researchers reported initially a slight increase in T3 and T4 concentrations induced by the restriction of REM sleep, followed by a significant decrease in the hormone concentrations (38, 39).
Sleep deprivation induces central hypothyroidism, which reduces TSH secretion and circulating T4 levels (32). Everson and Nowak assessed the HPT axis following sleep deprivation in rats and demonstrated reductions in serum T4 levels in sleep-deprived animals, but TSH was not dropped (15). They mentioned that central hypothyroidism induced by total sleep deprivation was attributed to a lack of TRH secretion. This function can be the same in sleep-deprived rats and sleep-restricted rats (32).
NE is one of the stress hormones used as an indicator of stressful situations in the body and this hormone plays an important role in adapting to stress (40-42). Plasma NE level depends on the activity rate of the sympathetic nervous system (43).
Studies reported that anxiety and depression are considerably related to increased NE levels in blood plasma (40, 43). Patients with depression disorder show increases in plasma NE concentration in different conditions (43, 44). However, in a study by Carney, no differences were found between depressed and non-depressed patients in either resting or standing plasma NE levels (45). As sleep-restricted rats showed higher mean NE levels than non-sleep restricted rats, it might show the amplification of the sympathetic system. This would partly describe increased energy costs (17).
5.1. Conclusions
In conclusion, our experiments, along with insights from earlier research, showed that exercise and sleep restriction could significantly reduce the weight of rats and indicated that sleep restriction increased depressive-like behavior and T4 plasma level. NE plasma level was found to be raised in sleep-restricted rats though it was not statistically significant. Our study also showed the changes induced by sleep restriction in plasma hormone levels, depressive-like behavior, and weight can be decreased by exercise. Since sleep deprivation is more happening in modern society leading to various neurobehavioral disorders, it seems it is important to identify appropriate ways to attenuate these effects. Our results showed that exercise can be a suitable candidate.