1. Background
Migraine is an episodic disorder that is believed to be due to a mixture of nervous factors and autonomic changes (1). The prevalence of migraine in women and men is 18.2% and 6.5%, respectively (2). In 90% of cases, migraine occurs up to age 40 years (3). Migraines involve unilateral headaches which last from 4 -72 hours, occur with moderate to severe intensity, are increased by physical activity, and can involve nausea, as well as sensitivity to light and sound (4, 5). It has been shown that factors such as dietary intake, time of day, hormonal changes, hunger, stress, family history, depression, and irregular sleep patterns are strongly associated with the incidence of migraine headaches (6).
Various factors are effective in the pathogenesis of migraines, but the main cause has yet to be known. Some of these theories refer to the role of nerve damage in the brain, as well as the density and pressure of brain nervous systems in migraine occurrence. Other factors that are thought to play a role include hyperhomocysteinemia, vitamin D deficiency, production of inflammatory factors and prostaglandins, production of serotonin from platelets, stimulation of norepinephrine production, and increased sensitivity to nitric oxide (7, 8).
Several previous studies have shown that some dietary components such as processed or fermented foods, chocolate, caffeine, salty foods, dairies, seafoods, citrus fruits, food additives (aspartame, monosodium glutamate, and nitrates), and tyramine-containing foods stimulate migraine attacks (6, 9, 10). On the other hand, it has been shown that specific nutrients, including magnesium, coenzyme Q10 (CoQ10), vitamin D, and omega-3 fatty acids, may improve the condition of these patients and decrease the severity and frequency of migraine attacks (11). Also, some studies have shown that adherence to vegetarian, low-fat, and elimination diets is effective in reducing the number and severity of migraine attacks, as well as decreasing the visual analog scale (VAS) (12).
Although several dietary factors have been associated with migraine, there are limited studies regarding the relationship between overall dietary pattern and migraine (13-15). Since foods influence each other, and it is difficult to study the effect of one specific food precisely, recently researchers have suggested dietary pattern analysis to investigate the relationship between nutritional intake and incidence of diseases (16, 17). Generally, dietary patterns can be considered as a network that involve the inter-relationship between different food items and dietary habits of a population. Successful strategies to treat or reduce the severity and frequency of headaches would confer substantial benefits to afflicted individuals.
2. Objectives
The present study aimed to evaluate the association between major dietary patterns and disease severity among patients with migraine.
3. Methods
3.1. Study Population
The present cross-sectional study included 256 female patients referred to two neurology clinics in Tehran, Iran, for episodic migraine diagnosis in 2016. More detailed information about the study population and migraine diagnosis has been provided in previous studies (18). In summary, we included females (18 - 50 years old) with BMI between 18.5 to 30 and diagnosis of migraine according to the International Classification of Headache Disorders 3 (ICHD3) criteria. The migraine was diagnosed by a neurologist, and all participants were referred to the neurology clinic for the first time. Subjects with diabetes, cardiovascular disease, cancer, and other chronic diseases and those using antihypertensive, anti-lipid, and anti-hyperglycemic drugs were excluded from the study. The methodology of this study was confirmed by the Ethics Committee of Tehran University of Medical Sciences, Iran (Project No. 95-01-103-31348).
3.2. Anthropometric Measurements
All participants were subjected to weight, height, waist circumference (WC), and hip circumference (HC) measurement. A digital scale (Seca) was used to measure weight with a precision of 100 gr. Also, a Seca stadiometer was used to measure the height of the participants with a precision of 0.1 cm. The BMI was obtained by dividing the weight (kg) by the square of height (m2). The WC and HC were assessed using a flexible tape with 0.5 cm accuracy. WC was measured between the iliac crest bone and the lowest rib. HC measurements were performed in the broadest area. WC was divided by HC to calculate waist to hip ratio (WHR). Anthropometric measurements were applied with the minimum cloth and without shoes.
3.3. Dietary Intake Assessment and Dietary Patterns
A 147-item semi-quantitative food frequency questionnaire (FFQ) was used to estimate dietary intakes in the last year (19). The reliability and validity of this questionnaire were approved in the Iranian adult population by Hosseini Esfahani et al. in a previous study (20). The FFQ data were analyzed by the Nutritionist-4 software.
3.4. Migraine Diagnosis
A neurologist diagnosed episodic migraine in participants according to the ICHD3 criteria (21). These criteria categorize episodic migraine into with aura and without aura types. Details for migraine characterization have previously been explained (18).
3.5. Migraine Severity Assessment
Participants were asked to complete a 30-day headache diary to collect the information of the time of migraine attack onset, duration of the headache, and headache frequency. The functional disabilities due to migraine in the last three months were assessed using the migraine disability assessment (MIDAS) tool (22). The reliability and validity of this questionnaire were confirmed in the Iranian population in a previous study (23). This questionnaire categorizes migraine patients into four groups according to the extent of the migraine effect on functionality. Grade I was assigned to participants who scored less than 5 (without or low disability), grade II to scores between 6 to 10 (mild disability), grade III to scores between 11 and 20 (moderate disability), and grade IV to scores more than 21 (severe disability). To assess the severity of headaches, a 10 cm VAS was used, and subjects were asked to rate their perception of pain (24). Participants with VAS scores between 1 and 3 were assigned to mild pain group, scores between 4 to 7 to moderate pain group, and scores more than 8 to severe pain group.
3.6. Statistical Analyses
Principal component analysis (PCA) was used to identify major dietary patterns. Due to the large number of food items in the FFQ, 23 food groups were created according to the similarity of their components (25). If the nutrient composition of a food item was significantly different from other food items or consuming it represented a certain food habit, that food could form a group by itself (such as tea and coffee, or egg). Migraine trigger foods (such as cheese, foods containing caffeine, various meats, and pickles due to their high levels of biological amines), foods containing additives, and processed foods were put into separate groups as much as possible (6, 21). However, segregating certain food groups (such as different kinds of vegetables and fruits) did not change the findings of the current study.
The varimax rotation was used to obtain a simple matrix to extract major dietary patterns with better interpretability. The suitability of data to perform PCA was assessed by the Anti-Image table, Kaiser-Meyer-Olkin Measure of Sampling Adequacy test, and Bartlett's Test of Sphericity. According to scree plots and the components’ interpretability, factors with eigenvalues ≥ 2.88 were extracted as major dietary patterns. The scores for each person were obtained based on the intake and loading factors of 23 different food groups for each of the two major dietary patterns (Table 1) (26). For simplicity, loading factors less than 0.3 are not shown in the table. After determining the adherence score to each of the two dietary patterns, the scores were converted into tertiles to perform an inter-tertile comparison. The Kolmogorov-Smirnov test was used to assess the normality of quantitative variables. To compare quantitative variables with normal or non-normal distribution across dietary patterns or migraine severity categories, the ANOVA or Kruskal-Wallis test was used. Also, to investigate the association between qualitative variables, the chi-square test was used. Multinomial logistic regression was performed to examine the relationship between dietary patterns as the independent variable with migraine outcomes as the dependent variable. Moreover, in the adjusted model of logistic regression, the effect of confounding variables (energy intake, BMI, the amount of water intake, and adding salt to food) was controlled. The SPSS software version 24 (IBM Corp. IBM SPSS Statistics for Windows, Armonk, NY) was used to analyze study data.
| Food Groups | Healthy Dietary Pattern | Unhealthy Dietary Pattern |
|---|---|---|
| High-energy drinks | 0.085 | |
| High-fat dairy | 0.812 | |
| Snacks and refreshments | 0.806 | |
| Pastries and sweet snacks | 0.796 | |
| Organ meats | 0.766 | |
| Low-fat dairy | 0.416 | -0.672 |
| Fast food | 0.608 | |
| Cheese | 0.438 | |
| Red meat | 0.434 | |
| Foods containing caffeine | -0.359 | |
| Pickles | -0.349 | |
| Fruits, fruit juices, and dried fruits | 0.738 | |
| Vegetables | 0.565 | -0.558 |
| Solid fats | -0.552 | 0.348 |
| Whole grains | 0.547 | |
| Liquid oil | 0.544 | |
| Refined grains | -0.511 | |
| Brains | 0.504 | 0.499 |
| Beans | 0.500 | |
| White meat | 0.409 | |
| Salt | -0.378 | |
| Egg | ||
| Spices and condiments | ||
| Percentage of variance explained | 14.51 | 24.53 |
| Total variances justified 39.05% |
Load Factor of Food Groups and the Percentage of Variance Explained by Each Dietary Patterna
4. Results
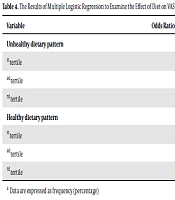
PCA resulted in two dietary patterns: unhealthy and healthy dietary patterns. In the unhealthy dietary pattern, while the intake of high-energy drinks, high-fat dairy, snacks, organ meats, processed foods, cheese, meat, nuts, and solid fats was higher, the intake of low-fat dairy, vegetables, foods with caffeine, and pickles was lower. In contrast, the healthy dietary pattern characterizes by a higher intake of fruits, vegetables, whole grains, vegetable oil, nuts, beans, low-fat dairy, white meat, and lower consumption of solid fats, refined grains, and salt. Factors that had a load factor greater than 0.3 are used to determine the type of dietary pattern. A load factor below 0.3 is omitted for naming the food pattern. These two factors explain 39.5% of the total variance overal. The load factors of food items available in each pattern are shown in Table 1. Negative loading factors show the inverse association, and positive values show the direct association between food items and dietary patterns. The relationship between the MIDAS and dietary patterns is given in Table 2. According to this table, MIDAS categories were not associated with healthy (OR = 0.81; 95% CI = 0.1, 62.05; P = 0.11) and unhealthy dietary pattern (OR = 0.88; 95% CI = 0.1, 69/13, P = 0.11). In Table 3, logistic regression did not show any significant relationship between healthy (OR = 0.75; 95% CI = 0.1, 56.01; P = 0.055) and unhealthy dietary patterns (OR = 0.89, 95% CI = 0.1, 66.19; P = 0.45) with MIDAS, after controlling for confounding variables. Table 4 provides the association between dietary patterns adherence and migraine headache severity (VAS). By the default suitability of odds ratio (OR), it is assumed that the difference in the OR does not vary from one category to the other. According to this table, the possibility of increasing the severity of migraine headaches is less in people in the first tertile of unhealthy dietary pattern versus people in the third tertile. This relationship also exists for the second tertile of unhealthy dietary pattern compared to the third tertile. The association of this unhealthy dietary pattern was significant with VAS at first before adjusting (OR = 0.53; 95% CI = 0.30, 0.93; P-trend = 0.02), but as shown in Table 5, after adjusting for underlying factors, no significant association was found (OR = 0.53; 95% CI = 0.23, 1.20; P-trend = 0.16). According to Table 5, in the unadjusted model of logistic regression, the association between VAS and the healthy dietary pattern was not statistically significant (P-trend = 0.14). However, the odds of severe migraine headache of subjects in 1st tertile of the healthy dietary pattern were 1.82 (95% CI = 0.96, 3.44) times that of the subjects in the last tertile, after adjusting for the effect of confounding variables (P-trend = 0.02).
| OR (95%CI) | P-Value | |
|---|---|---|
| Unhealthy dietary pattern | 0.88 (0.1 - 69.13) | 0.32 |
| Healthy dietary pattern | 0.81 (0.1 - 62.05) | 0.11 |
The Results of Multiple Logistic Regressions to Examine the Effect of Diet on MIDAS (Severe Disability- Non-Severe Disability)
| Odds Ratio (95% Confidence Interval) | P-Value | Odds Ratio (95% Confidence Interval) | P-Value | ||
|---|---|---|---|---|---|
| Unhealthy dietary pattern | 0.89 (0.1 - 66.19) | 0.45 | Healthy dietary pattern | 0.75 (0.1 - 56.01) | 0.055 |
| Energy intake | 1.07 (0.1 - 80.42) | 0.63 | Energy intake | 1.03 (0.1 - 77.37) | 0.80 |
| BMI | 1.26 (1.1 - 17.35) | < 0.000 | BMI | 1.27 (1.1 - 18.36) | < 0.001 |
| Water consumption | 0.93 (0.1 - 85.02) | 0.15 | Water consumption | 0.94 (0.1 - 85.03) | 0.20 |
| Adding salt to food | 1.32 (0.2 - 72.44) | 0.36 | Adding salt to food | 0.73 (0.1 - 39.36) | 0.33 |
The Results of Multiple Logistic Regression to Examine the Effect of Dietary Pattern on MIDAS After Considering the Confounding Factors
| Variable | Odds Ratio (95% Confidence Interval) | P-Value | P-Trend |
|---|---|---|---|
| Unhealthy dietary pattern | 0.02 | ||
| 1st tertile | 0.53 (0.30 - 0.93) | 0.02 | |
| 2nd tertile | 0.47 (0.27 - 0.84) | 0.01 | |
| 3rd tertile | Reference group | ||
| Healthy dietary pattern | 0.14 | ||
| 1st tertile | 1.50 (0.87 - 2.68) | 0.14 | |
| 2nd tertile | 1.06 (0.61 - 1.85) | 0.82 | |
| 3rd tertile | Reference group |
The Results of Multiple Logistic Regression to Examine the Effect of Diet on VASa
| OR (95% CI) | P-Value | P - Trend | OR (95% CI) | P-Value | P-Trend | ||
|---|---|---|---|---|---|---|---|
| Unhealthy dietary pattern | 0.16 | Healthy dietary pattern | 0.02 | ||||
| 1st tertile | 0.53 (0.23 - 1.20) | 0.12 | 1st tertile | 1.82 (0.96 - 3.34) | 0.06 | ||
| 2nd tertile | 0.55 (0.26 - 1.14) | 0.11 | 2nd tertile | 1.38 (0.74 - 2.57) | 0.30 | ||
| 3rd tertile | Reference | 3rd tertile | Reference | ||||
| Energy intake | 1.61 (1.14 - 2.26) | 0.006 | Energy intake | 1.84 (1.40 - 2.43) | < 0.001 | ||
| BMI | 1.20 (1.12 - 1.28) | < 0.001 | BMI | 1.20(1.12 - 1.28) | < 0.001 | ||
| Water consumption | 0.89 (0.80 - 0.99) | 0.03 | Water consumption | 0.88(0.79 - 0.98) | 0.02 | ||
| Adding salt to food | 4.20 (2.26 - 7.79) | < 0.001 | Adding salt to food | 4.34(2.27 - 8.33) | < 0.001 | ||
The Results of Multiple Logistic Regression to Examine the Effect of Dietary Pattern on VAS after Considering the Confounding Factors
5. Discussion
The present study investigated the association between major dietary patterns and disease severity in women with migraine. Generally, two major dietary patterns, including the healthy and unhealthy dietary patterns, were identified. The results of the study revealed that more adherence to the healthy dietary pattern is associated with lower severity in migraine headaches. Although there was a direct association between VAS and following the unhealthy dietary pattern, this relationship was confounded by other variables. Moreover, this study did not find any association between migraine disability and dietary patterns.
Rezazadeh et al. studied women aged 20 - 50 in Tehran, Iran, and found two dietary patterns: healthy and unhealthy (27). In another study conducted on Tehranian adults, three patterns were identified: western, healthy, and mixed. The western and healthy dietary patterns were similar to unhealthy and healthy dietary patterns in our study, respectively (28). Also, there was some similarity between the healthy dietary pattern in the present study with other studies in the adult population globally (29, 30). On the other hand, there are many commonalities between the unhealthy dietary pattern in the present study and the western dietary pattern in other studies (29, 31). It should be noted that dietary patterns are comparable if the food group classification is exactly the same (32).
Also, the research topic can play a role in categorizing dietary patterns, which can then cause differences in dietary pattern classification (33). Since all the subjects of the current study had migraine, being in this situation could affect their dietary patterns. This was shown in a study carried out on the relationship between dietary patterns and migraine by Rist et al. (34), which observed lower intakes of caffeinated coffee in subjects with non-migraine headaches but did not observe this in migraine patients. Additionally, this group’s consumption of chocolate and burgers was greater than subjects without headaches. On the other hand, migraine patients consumed less dairy products than non-migraine ones. However, this study showed that dietary patterns of people with migraine are different in a healthy society; this could be attributed to the fact that migraine patients change their dietary patterns over time due to headaches being caused by certain foods. One study revealed that among normal-weight people (BMI between 18.5 to 24.5), the patients with migraines had lower food quality. Lower intakes of whole grains, fruits, vegetables, beans, and legumes were reported in women diagnosed with migraine compared to healthy counterparts (30). This suggests that dietary patterns obtained from women with migraine may be different from those of healthy subjects. In this study, it was assumed that adherence to different dietary patterns (healthy and unhealthy) could have a significant correlation with the severity of migraines. According to the results of the study, adherence to the unhealthy dietary pattern before and after controlling for confounding variables does not have a significant effect on MIDAS, and as a result, this hypothesis is rejected.
Vegetables intake was lower in the unhealthy dietary pattern. It has been suggested that there is an active agent in some vegetables, including cabbage, broccoli, spinach, beets, parsley, and carrots, that may act as an antagonist for calcitonin gene-related peptide (CGRP), a protein responsible for intense inflammation in the meninges. Therefore, higher intakes of this agent may prevent migraine attacks, and in some cases, it has been reported to be more effective compared to anti-migraine medications (35). Of the other foods that can be introduced as a migraine trigger, fermented and processed foods, pickles, and seafood can be named. Monosodium glutamate is one of the factors which stimulates migraine. The histamine in seafood, meats, and dairy products, and tyramine in different foods (such as red wine, strong or aged cheeses, smoked fish, some fruits, certain beans, and onions) can contribute to triggering migraines. In addition, caffeine, alcohol, and nitrates in meat products are also effective in creating headaches (36). This non-significant association between adherence to a healthy dietary pattern and migraine disability could be attributed to the fact that migraine can be stimulated by some foods in a healthy diet. For example, foods such as seafood, dairy products, figs, beans, citrus, avocados, bananas, and onions, which are involved in triggering migraines, are placed in the healthy diet. As a result, the effect of beneficial foods may be lost with the simultaneous consumption of foods that are migraine drivers. However, in the present study, foods that are known to be migraine drivers in various studies were categorized into separate groups at the primary analysis stage. However, there was no difference in the study results.
Studies have shown changes in the dietary patterns of migraine sufferers over time due to their problems (36). Dietary changes can also occur with taking drugs. These changes have been observed particularly when receiving topiramate, valproic acid, and tricyclic antidepressants (37, 38). As a result, people who have more severe migraines and use more drugs may have more dietary changes.
5.1. Conclusions
Overall, our results showed that the unhealthy dietary pattern may affect the severity of migraine headaches, but this relationship is influenced by other confounding factors, too. Also, the healthy dietary pattern after controlling for confounding variables reduced the severity of pain. In this study, there was no significant relationship between food patterns and the disability of migraine. According to the explanations given, migraine can change the dietary intake and eventually affect the association between migraine disease severity and dietary pattern in scientific studies. In addition, relying on self-reported data could also have a high impact on this relationship. The lack of correlation between other indicators of migraine severity with food patterns may be due to the presence of foods in the healthy diet that normally trigger a migraine and thus neutralize the beneficial effects of this food pattern. In general, following a healthy diet and lower consumption of unhealthy foods by modifying these diets and eliminating stimulants may play a significant role in migraine outcomes; however, this relationship must be confirmed in future studies.