1. Background
Spinal cord injury (SCI) is a disabling and irreversible condition that involves both primary and secondary injury mechanisms of damage. The primary mechanism is a mechanical injury that initiates a cascade of secondary injury mechanisms (1, 2). Numerous studies reported that prolonged compression of the spinal cord is associated with significant physiological, histological, and genetic alterations at the cellular level (2, 3). These may be associated with pathogenic auto-destructive processes such as hemorrhage, apoptosis, glutamate excitotoxicity, inflammatory/immune activation, demyelination, and reactive gliosis (4). Such alterations, along with inhibitory factors released by damaged myelin and reactive astrocytes, may contribute to the death of neurons, oligodendroglial cells, and inhibition of axon outgrowth (5). The nature and amplitude of these alterations depend on the severity of the initial injury and have an important role in determining the appropriate interventions (6).
Among the inhibitory factors, RhoA, an intracellular GTPase, that prevent neuroregeneration in SCI, is considered as a target to design therapeutic strategies (7). It's proved that Rho is a major intracellular effector for axonal growth inhibition, mainly through inhibitory molecules derived from central nervous system (CNS) myelin and reactive astrocytes (8). Besides, it's demonstrated that oligodendrocyte myelin-associated glycoprotein (MAG), Nogo, and chondroitin sulfate proteoglycans (CSPGs) expressed by reactive astrocytes can activate RhoA, which in turn causes inhibition of neurite outgrowth from neurons (9). It has been shown recently that RhoA signaling influences the inhibition of neurite growth via the p75 neurotrophin receptor (p75NTR) (10). Indeed, many studies indicated that p75NTR -null mutant mice (10) are not inhibited by MAG, and RhoA binding to p75NTR forms part of the raft receptor complexes that triggers growth inhibition signaling cascade (11).
The p75NTR, which expresses by developing neurons as well as injured neurons, mediates cell survival or apoptosis (12). Also, Rho activation can induce apoptosis via p75NTR (13). The SCI influences the expression level of the neurotrophin receptor, and its level may be higher at the border between the lesion and intact spinal cord tissue (9, 14). S100β is a calcium-binding protein produced by the CNS (15) and the spinal cord. It mostly appears in astrocytes. It is also produced by Schwann cells in the peripheral nervous system (PNS), where it regulates the cytoskeletal structure and cell proliferation (16). S100β has a neurotrophic activity for neural cells during the development and regeneration activity after injury. S100β also stimulates the expression level of pro-inflammatory factors and initiates apoptosis processes (17). Based on what was mentioned before, evaluation of SCI severity by identifying related molecules would be useful for determining potential therapeutic targets intended to prevent or reduce secondary injuries.
2. Objectives
According to the best knowledge of the authors, no study has investigated the association between severity of SCI and expression levels of RhoA, p75NTR, and S100β in spinal cord injury. The current study aimed to evaluate the changes in expression levels of RhoA, p75NTR, and S100β in the short and prolonged compression of the spinal cord in a compressive model SCI.
3. Methods
3.1. Animals
Adult female Wistar rats weighing 200 ± 280 g were obtained from an in-house animal facility at Tehran University of Medical Sciences. Scientific procedures and the welfare of animals were observed following the guideline developed by the Sina Trauma and Surgery Research Center (affiliated to the Tehran University of Medical Sciences (TUMS)). All animals were housed in groups of four at an ambient temperature of 22 ± 1°C with a 12-h light and 12-h dark cycle. The rats were divided into three groups. Sixteen rats were injured using aneurism clip compression for 3 seconds (short compression), 16 rats were injured using aneurysm clip compression for 10 minutes (prolonged compression), and six rats underwent a sham operation (only laminectomy).
3.2. Compression Model
The rats were deeply anesthetized by injecting a mix of ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally. A skin incision was made, fascia and paravertebral muscles were dissected to expose the T9 vertebrae lamina, and the spinal cord was completely compressed over its diameter by extradural application of a 134g aneurysm clip (AESCULAP1 MINI-CLIP) at the T9 level for either 3 seconds (short compression) or 10 minutes (prolonged compression). Then, the skin was sutured, 5 ml of saline was injected subcutaneously, and animals were kept under a heat lamp to reestablish body temperature. Gentamicin (1 mg/kg) and cefazolin (75 mg/kg) were administered once daily. Rats that exhibited complete hindlimb paralysis 24 h after injury were excluded from the study (n = 3). Animals were monitored for autophagia, and their bladder was manually expressed.
3.3. Histological Evaluation
At 6 hours, 1 day, 3 days, 7 days, and 14 days after injury, rats were perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.4) transcardially (3 rats for each time point). The spinal cords were dissected from 1 cm rostral and 1 cm caudal to injury epicenter (2 cm total length), post-fixed overnight in 4% paraformaldehyde, and embedded in paraffin for transverse sectioning. The spinal cords were sectioned at 5 μm thickness. Tissue sections were stained with cresyl violet.
3.4. Immunohistochemistry
The sections were deparaffinized, rehydrated, and incubated in blocking solutions (5% normal goat serum, 2% BSA, and 0.3% Triton X-100 in 0.01 M PBS) at room temperature for one hour. Then, the sections were incubated with primary antibodies, including rabbit anti-p75 antibodies (1:100, Abcam, Cambridge, MA, USA), anti- S100β antibody (1 1:100, Abcam, Cambridge, MA, USA), and anti-RhoA antibody (1:100, Abcam, Cambridge, MA, USA), overnight at 4°C in a humidified chamber. After performing 2 washes in PBS, the sections were incubated in FITC-conjugated IgG secondary monoclonal antibody (1:100, Abcam, Cambridge, MA, USA) for 1 hour at 37ºC, and the nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI) (1:1,000) solution. Immunostaining was detected under a fluorescent microscope. The optical density of fluorescence was analyzed using ImageJ software.
3.5. Locomotor Functional Testing
Locomotor function after SCI was assessed in an open field (diameter of 50 cm) for 4 min at a similar time of day for each testing using Basso, Beattie, and Bresnahan Locomotor Rating Scale (BBB) (18). Two blinded evaluators scored hindlimb locomotor function in each animal per week for 2 weeks after injury. The scale measures a range of BBB scale, from complete paralysis of the hindlimbs (Score: 0) to normal walking behavior (score: 21), by assessing hind limb joint movements, stepping, trunk position and stability, coordination of forelimb and hindlimb, paw placement, toe clearance, and tail position.
3.6. Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM). Statistical analyses were performed with Graph Pad Prism version 5.0. For multiple comparisons, the ANOVA test with a post hoc Tukey's test was used. Statistical significance between the two groups was determined by Student's t-test. Statistical significance was considered when P-value < 0.05.
4. Results
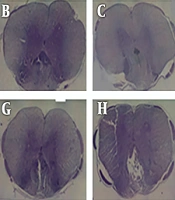
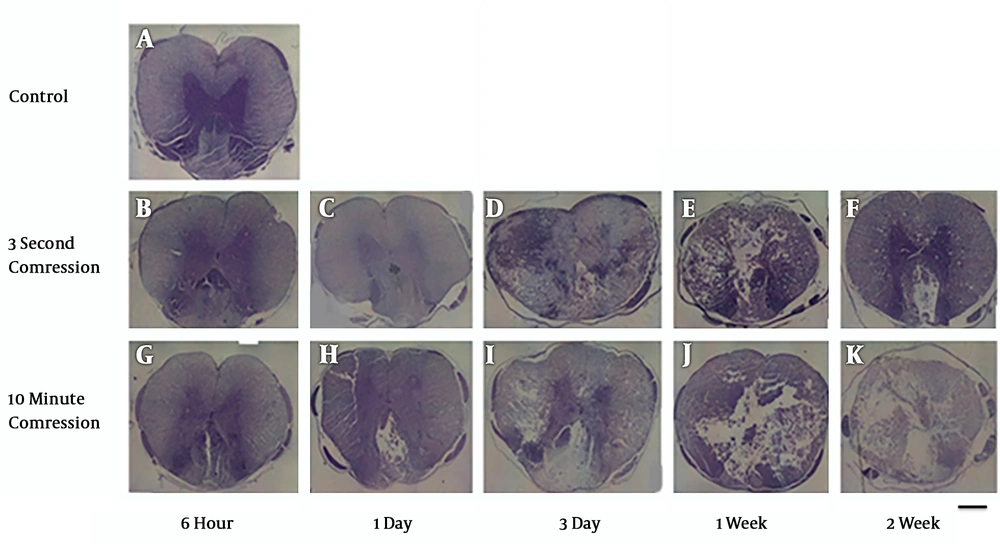
Cresyl violet staining was used to assess spared tissue and lesion size. The results indicated differences concerning the lesion size in the control group compared with those of the experimental group (Figure 1). The lesion area in the injured spinal cord was evaluated using light microscopy. The lesion areas in the animals injured through 3-second compression were smaller than those in the 10-minute compression group when assayed at days 3 and 14 after injury. Besides, it was found that the lesion area was increased at day 14 following compressive injury in all three groups, but the lesion areas in the severely injured animals were larger compared to those in the mildly injured animals.
Cavity formation gradually increases over time following spinal cord compression at the 5 mm rostral to the injury site. Light microscopic evaluation of cresyl violet staining of the spinal cord sections at 3- and 14-days post-SCI shows greater cyst cavity in the 10-minute compression group compared to the 3-second compression group. (scale bar: 100µm). A: control group, B: 3second- 6hour, C: 3second- 1day, D: 3second- 3day, E; 3second-1week, F: 3second- 2week, G: 10minute-6hour, H: 10minute-1day, I: 10minute-3day, J: 10minute-1week, K: 10minute- 2week.
4.1. Effect of Time-Dependent Compression on the Expression of p75NTR
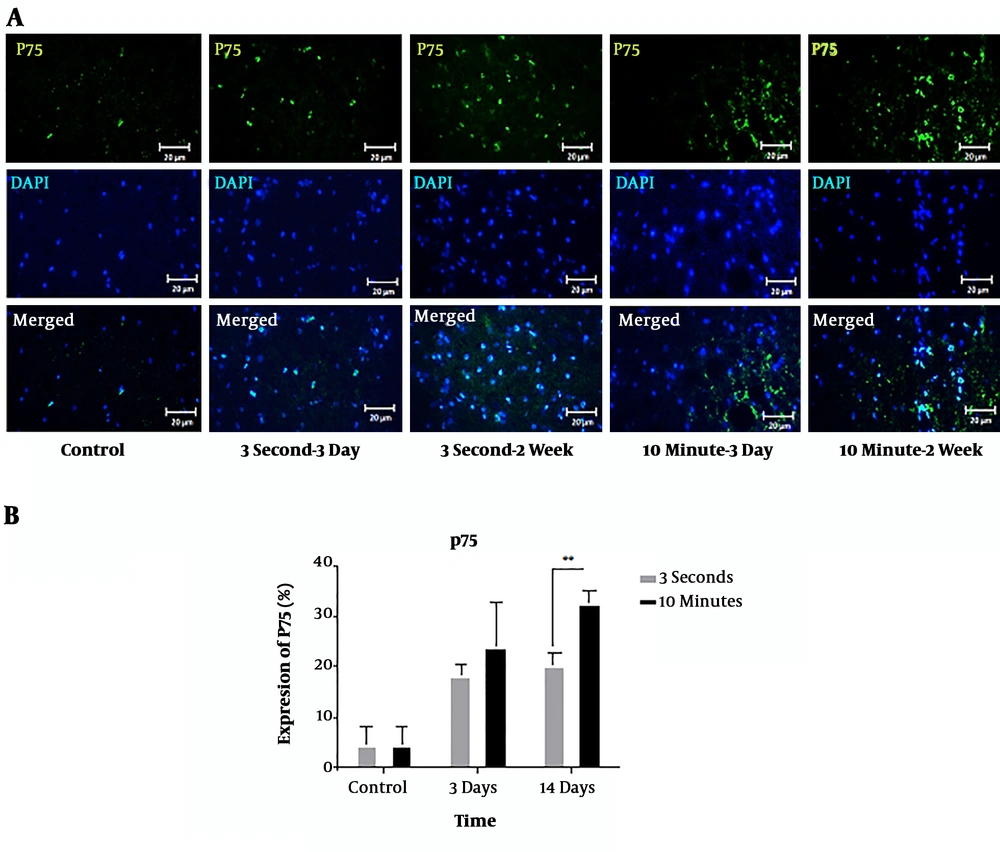
The extent of the p75NTR expression in cord tissues injured (induced by 3-second and 10-minute compression) was evaluated by immunohistochemistry (Figure 2A). P75NTR was significantly overexpressed at the 5 mm rostral to the injury site in both severities of injury at 3 and 14 days following the injury compared to the sham-operated animals. Ten-minute compression also caused a greater expression level of p75NTR at the level of injury compared to 3-second compression. At 14 days after injury, a significant difference was found between p75NTR expression in two injury groups (P < 0.01). However, 3 days after injury, the difference between the two injury groups was not statistically significant (Figure 2B).
Immunohistochemical staining of p75NTR protein following 3-second and 10-minute compression at the 5 mm rostral to the injury site. A) Immunostaining for p75 revealed various amounts in the spinal cord sections of T9 in the different groups at 3- and 14-days post-SCI. p75-positive cells are stained green, while the nuclei are stained with DAPI (blue) (20× magnification) (scale bar: 20 µm). B) Comparison of p75NTR expression changes at the 5 mm rostral to the injury site. The staining intensity in the 10-minute compression group is higher than in the 3-second compression group after 14 days and the control group. Data are expressed as the mean ± SEM. Analysis of variance was used for statistical analysis (n = 3). ** P < 0.01.
4.2. The Influence of Time-Dependent Compression on the Expression of S100β
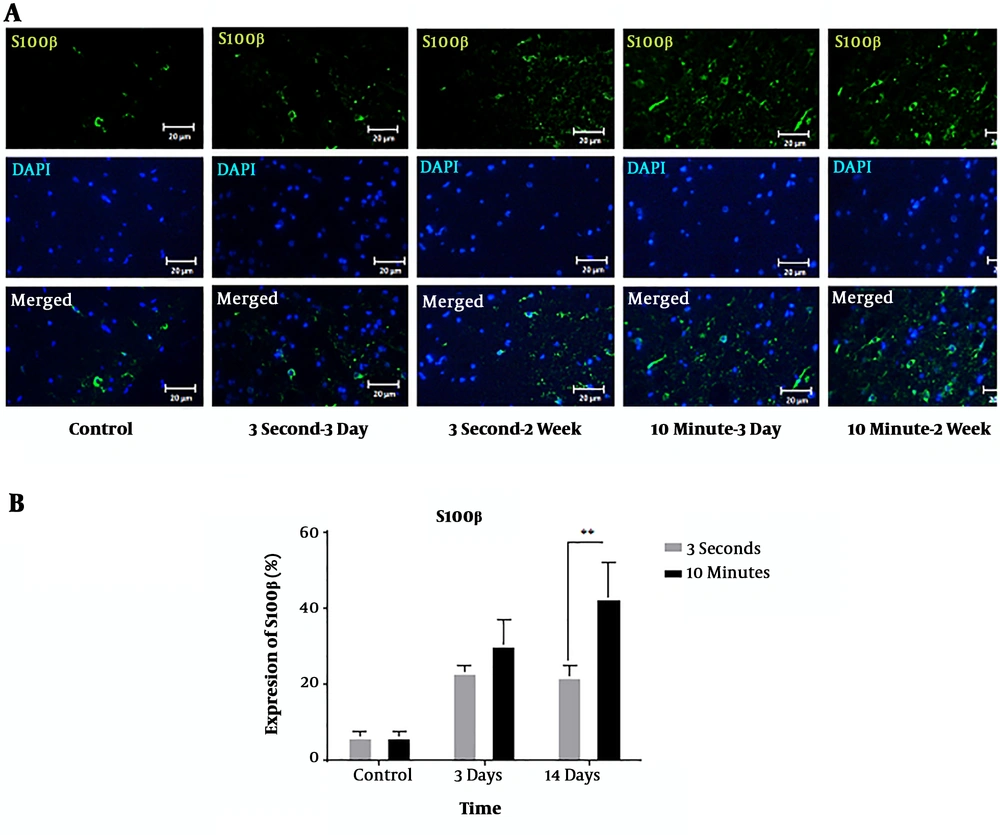
According to the findings, the SCI increased the expression level of S100β at 3 and 14 days after injury compared to the sham-operated animals (Figure 3A). The expression level after 10-minute compression was higher than those with 3-second compression. At 14 days, there was a statistically significant difference between S100β expressions in the two groups (P < 0.01). Three days after decompression, there was no significant difference between the two models (Figure 3B).
Immunohistochemical staining of S100β protein following 3-second and 10-minute compression at the 5 mm rostral to the injury site. A) Immunostaining for S100β revealed various amounts in the spinal cord sections of T9 in the different groups at 3- and 14-days post-SCI. S100β-positive cells are stained green, while the nuclei are stained with DAPI (blue) (20 × magnification) (scale bar: 20 µm). B) Comparison of S100 expression changes at the 5 mm rostral to the injury site. The staining intensity in the 10-minute compression group is higher than in the 3-second compression group after 14 days and the control group. Data are expressed as the mean ± SEM. Analysis of variance was used for statistical analysis (n = 3). ** P < 0.01.
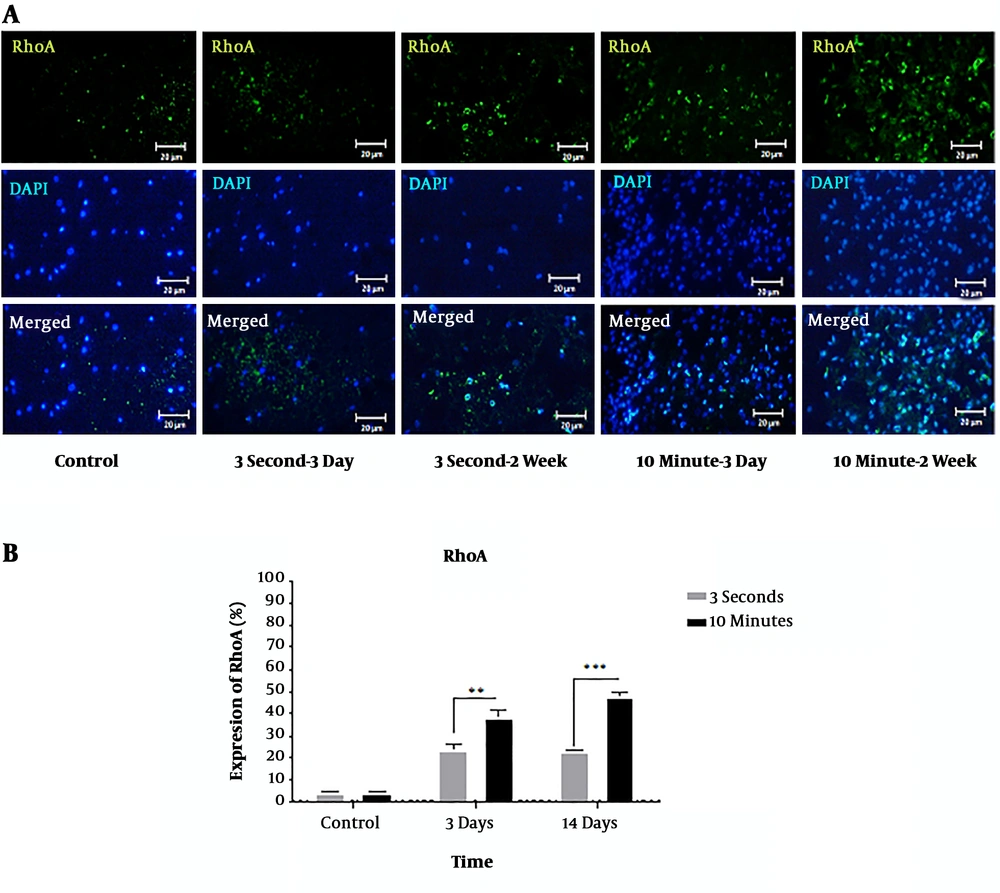
4.3. The Influence of Time-Dependent Compression on the Expression of RhoA
RhoA was significantly overexpressed at the 5 mm rostral to the injury site in the 3-second and 10-minute compression models at 3 and 14 days after the injury compared to the control group. At both 3 and 14 days, there was a statistically significant difference between the RhoA expressions in the two models (P < 0.01, P < 0.001) (Figure 4A and B).
Immunohistochemical staining of RhoA protein following 3-second and 10-minute compression at the 5 mm rostral to the injury site. A) Immunostaining for RhoA revealed various amounts in the spinal cord sections of T9 in the different groups at 3- and 14-days post-SCI. RhoA-positive cells are stained green, while the nuclei are stained with DAPI (blue) (20× magnification) (scale bar: 20 µm). B) Comparison of RhoA expression changes at the 5 mm rostral to the injury site. The staining intensity in the 10-minute compression group is higher than in the 3-second compression group after both 3 days and 14 days and the control group. Data are expressed as the mean ± SEM. Analysis of variance was used for statistical analysis (n = 3). ** P < 0.01, *** P < 0.001.
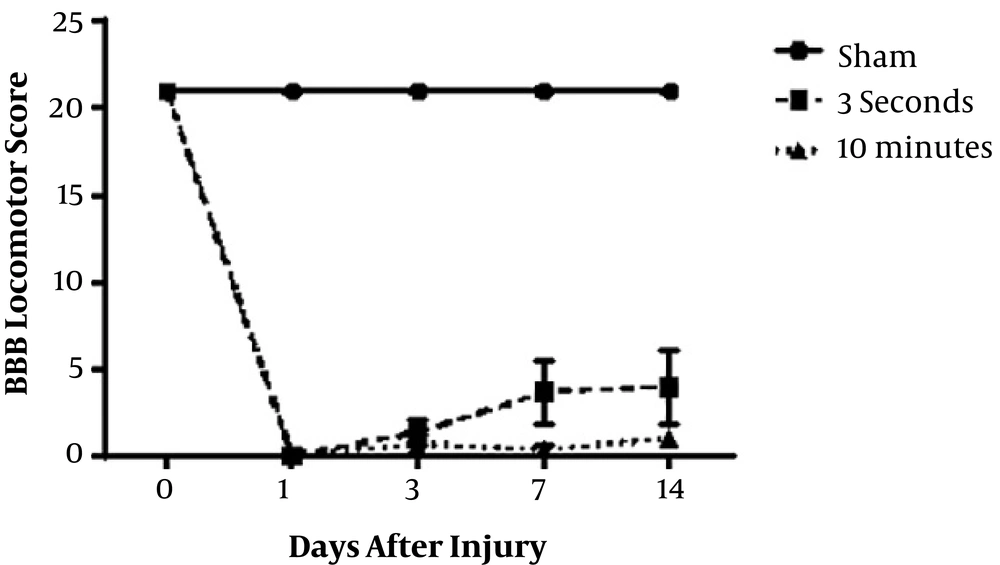
4.4. Locomotor Functional Recovery
To assess the locomotor recovery, BBB open field was performed on the 1, 3, 7, and 14 days (Figure 5). Animals had no hindlimb movement immediately post-injury (score of 0). After the injury, hind-limb locomotion was decreased significantly in all animals in both experimental groups compared to the control group. Spontaneous improvement in locomotor function occurred slightly from 3 days in the group that received 3-second compression.
Locomotion function was compared in 3-seconds and 10-minutes spinal cord compression groups according to the Basso, Beattie, and Bresnahan (BBB) score. After injury, hind-limb locomotion was lost in all animals in both groups. Spontaneous improvement in locomotor function has been started in 3-second compression group three days after decompression (n = 3).
5. Discussion
Investigating the effect of compression duration on the expression changes of molecules that contribute to the cascade of secondary injury following initial impact can provide useful information for developing novel clinical interventions intended to treat the dysfunction. Primary injury to the spinal cord stimulates a series of downstream cellular responses, which leads to a secondary injury that induces alterations in protein expression of neurons and other cells (4, 19). The persistent compression or displacement can induce neurological dysfunction by interrupting the blood flow, secretion of free radicals, inflammation, and apoptosis (20). The severity of injury affects the grade of gene expression. It's well-documented that growth inhibitory proteins that release reactive astrocyte and damaged myelin, such as CSPGs and MAG, can suppress axonal regrowth in cases with SCI (21, 22). Previous studies have shown that CSPGs and MAG active RhoA in both neurons and glial cells in a p75NTR dependent manner (23). Several studies reported that injury to the spinal cord stimulates the expression of RhoA, p75NTR, and S100, which in turn triggers apoptosis and inflammatory cascades that leads to the death of neurons and glial in the spinal cord and inhibit axonal regeneration (14, 24-28).
In the present study, a clip impact-compression model of SCI was used to evaluate the effect of duration of compression on the expression of RhoA, p75NTR, and S100. At the 5 mm rostral to the injury site, we found that in prolonged compression expression of p75NTR, S100 was significantly upregulated compared to short compression and control groups. Based on the findings, p75NTR expression was upregulated at both days 3 and 14 after short and prolonged compression of the spinal cord. Brunello et al. showed that SCI produces a remarkable and rapid increase in p75NTR mRNA in the tissue close to the injury site (24). Montazeri et al. reported temporal and spatial patterns of alteration in p75NTR expression levels following SCI. They showed that p75NTR expression begun six hours after SCI, and its increase continued to seven days and then reduced to 10 days after injury. Alterations in the expression level of p75NTR in the spinal cord damage following injury play a pivotal role in apoptotic cell death (29). In another study, which used a spinal cord compression model, Casha et al. demonstrated enhancement of p75NTR expression in oligodendrocytes, microglia, and astrocytes following SCI (30). Also, a study, which used a contusion model, showed that spinal cord damage resulted in a significant increase of S100β immunoreactive area at 72 hours, 1 week, and 3 weeks (26).
RhoA expression was also upregulated in 10-minute and 3-second compression models of SCI at 3 and 14 days compared to the control group. The prolonged compression induces a further increase in RhoA expression. There was a significant difference in the RhoA expression at 3 and 14 days in the 10-minute and 3-second compression model. Both RhoA mRNA and RhoA are significantly higher in neurons and glial cells after SCI near the injury site compared to the normal control spinal cord (13). The increased RhoA expression in the neurons and glial cells has important roles in inhibiting neural regeneration in the first week after SCI (31). In this study, histologic evaluation of the spinal cord of rats demonstrated similar specific changes with our previous studies, that increasing duration of compression can intensify the damage volume and cavitation area that correlated with the BBB locomotor scores (32).
Some studies reported that prolonged compression worsens neurological recovery and early decompression improves functional recovery (33). Earlier surgical decompression is an important concern in achieving better neurological outcomes following traumatic SCI. Besides, it also dampens secondary injury mechanisms (2, 3). These findings are in agreement with our previous studies that subjects with 3-second spinal cord compression had better final neurological recovery and experienced decreased lesion volumes compared to the 10-minute compression group (34). Dolan et al. used a clip compression injury model at C7-T1 to investigate the effect of time until decompression, ranging from 15 to 240 minutes. They found prompt relief of persistent compression was associated with improved neurological recovery after different compression forces (35). Besides, it was associated with lower levels of edema, less myelin and axon damage, and more myelin regeneration in rats in the 3-second compression group compared to the 10-minute compression group (36).
5.1. Conclusion
In conclusion, this study demonstrated that prolonged compression of the spinal cord could induce changes in RhoA, p75, and S100 expression, which were significantly different from changes that usually occur after short compression. The results suggest that increasing RhoA, p75, and S100 expression during the compression of the spinal cord plays an important role in the molecular cascade of secondary injury. In other words, these results show that early decompression of the spinal cord may modulate secondary injury events by causing changes in RhoA, p75NTR, and S100β expression. The current study had limitations, such as not performing a cresyl violet staining or immunohistochemical of the tissues in the epicenter of the injured area, which probably has affected verification of the efficacy of the spinal cord models.