1. Background
Lumbar disc herniation is one of the most frequent surgically-treated disorders of the lumbar spine (1, 2). However, discectomy-related complications occur in 15 - 30% of patients (3-5).
Dexmedetomidine is an alpha 2 adrenergic agonist that is used in the operation theatre and intensive care unit to achieve sedation, analgesia, and anxiolysis. Dexmedetomidine is known for its sedative properties and desired effects in achieving more ventilator-free hours in the intensive care unit (6). Compared to benzodiazepines, it has been proven that intravenous dexmedetomidine prevents delirium among critically ill patients (7). Other favorable characteristics of dexmedetomidine seem to be improved survival among elderly patients and better cognitive function in patients who survive more than three years (8). Moreover, the protective effects of dexmedetomidine on apoptosis and necrosis of the brain and other tissues have been reported (9).
2. Objectives
Some of the important apoptotic factors evaluated in this study included Bax/Bcl-2 and caspase-3 in human subjects. The evidence of bio-molecular changes in Bax/Bcl-2 and caspase-3 expressions is less clear. Accordingly, this study aimed to evaluate the effects of intrathecal dexmedetomidine administration on the CSF levels of apoptotic factors in patients undergoing lumbar discectomy surgery.
3. Methods
3.1. Study Population
The study population was 20 to 60 years of age people scheduled for lumbar discectomy surgery (Table 1). Of 44 enrolled cases, one case did not have informed consent, two cases were eliminated due to blood transfusion, and one case missed CSF analysis; so, we evaluated 40 cases by the end of the study (Figure 1).
| Variables | Groups | P-Value | ||
|---|---|---|---|---|
| Intervention | Control | Total | ||
| Age (y) | 41.7 ± 10.1 | 41.4 ± 7.2 | 41.5 ± 8.6 | 0.16 b |
| Gender | 0.74 c | |||
| Male | 12 (60) | 11 (55) | 23 (57.5) | |
| Female | 8 (40) | 9 (45) | 17 (42.5) | |
| Discectomy level | 0.31 c | |||
| I | 12 (60) | 15 (75) | 27 (67.5) | |
| II | 8 (40) | 5 (25) | 13 (36.5) | |
| Blood pressure | 1.0 c | |||
| Changed | 1 (5) | 1 (5) | 2 (5) | |
| Not changed | 19 (95) | 19 (95) | 38 (95) | |
| Heart rate | 0.21 c | |||
| Changed | 2 (10) | 5 (25) | 7 (17.5) | |
| Not changed | 18 (90) | 15 (75) | 33 (82.5) | |
| Surgery duration (min) | 111.0 ± 28.4 | 105.7 ± 23.7 | 108.8 ± 26.1 | 0.33 b |
| Numerical rating scale | 3.7 ± 1.3 | 5.1 ± 1.3 | 4.8 ± 1.2 | 0.004 b |
| Hemorrhage (cc) | 467.5 ± 130.0 | 430.0 ± 120.7 | 448.2 ± 125.3 | 0.36 b |
Age, Gender, and Clinical Data in Two Groups a
3.2. Inclusion and Exclusion Criteria
The inclusion criteria included: (1) patients in the age group of 20 to 60 years who were candidates for lumbar discectomy surgery; (2) no history of receiving anti-inflammatory drugs within two weeks before surgery; (3) no immunodeficiency or neurodegenerative diseases; (4) no history of systemic diseases such as diabetes mellitus, renal failure, uremia, hepatic disorders, chronic cardiopulmonary disease, or myopathy; and (5) informed consent. The exclusion criteria included: (1) a change in the approach of surgery; (2) the need for transfusion during surgery; and (3) the duration of surgery of more than four hours.
3.3. Measurements
The present study was performed as a clinical trial on patients with lumbar disc herniation who went through discectomy surgery. After obtaining permission from the ethics committee of Shahid Beheshti University of Medical Sciences and selecting the patients to participate in the study, the researchers obtained informed consent. The study subjects were randomly divided into control and dexmedetomidine groups based on the random numbers table. Medical staff and researchers from the beginning of the study closely monitored patients in both groups. The treatment and care protocols were implemented completely and identically in both groups.
A quantitative NRS system was used to assess the level of pain. The induction of anesthesia in all patients was the same using 3 μg/kg of fentanyl, 0.02 mg/kg of midazolam, and 1.5 mg/kg of lidocaine as premedication and 2 mg/kg of propofol and 0.5 mg/kg of atracurium as induction agents, and arterial line established to monitor blood pressure. With the patient in a prone position and following the surgical incision and reaching the dura, the surgeon took a one-milliliter sample of the CSF using a 27-gauge spinal needle.
The spinal needle in the dexmedetomidine group remained in place, and the syringe was removed. Then, 0.1 μg/kg of dexmedetomidine was injected into the intrathecal space from the same needle, but in the control group, the needle was removed after sampling. The sampling was performed again at the end of the discectomy, and 1 cc of the CSF was taken again in both groups, according to the reference method. During the surgery, hemodynamic parameters (heart rate and mean arterial pressure) were monitored. In the recovery room, the patients' pain scores were measured by the NRS. After centrifugation at 448 G for 10 minutes, CSF samples were frozen immediately at -70°C. The collected samples were sent to the laboratory of the medical school, where the apoptotic factors, Bax/Bcl-2 and caspase-3 (Zellbio, Germany), were measured by the enzyme-linked immunosorbent assay. The same surgeon performed the entire study process for all patients, including surgery, CSF sampling, and drug injection.
3.4. Ethical Considerations
The study group adhered to the principles of medical ethics introduced by the Health Ministry, the Declaration of Helsinki, and legislations in the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences. In addition, the Ethics Committee of Shahid Beheshti University of Medical Sciences approved the protocol of the study. The cost of used medications was supplied by research resources. This investigation did not have any effect on the patients’ treatment process, and the result of the assessment was only reported to the patient later. The codes were as follows: Ethical code, IR.SBMU.MSP.REC.1398.098, IRCT code: IRCT20200127046282N1.
3.5 Statistical Analysis
The data were analyzed by SPSS version 23, and P < 0.05 was considered significant. Means ± SD were used to present descriptive results of quantitative variables. Ratios and percentages were used to present descriptive results of qualitative variables. Also, a paired t-test and ANOVA test were used for quantitative variable analysis if normality and equality of variances were established, and Wilcoxon and Friedman's tests were used if these test conditions were not met.
4. Results
4.1. Clinical Characteristics
Demographic data were not statistically different (Table 1). Also, there were no statistically significant differences in clinical data, including discectomy level (P = 0.31), blood pressure change (P = 1.0), heart rate change (P = 0.21), surgery duration (P = 0.33), and hemorrhage volume (P = 0.36) (Table 1).
4.2. Numeric Rating Scale
Using the NRS as a pain scale, the scores of the dexmedetomidine and control groups were 3.7 ± 1.3 and 5.1 ± 1.3, respectively; based on an independent sample t test, there was a statistically significant difference (P = 0.004) (Figure 1).
4.3. CSF Apoptotic Factors
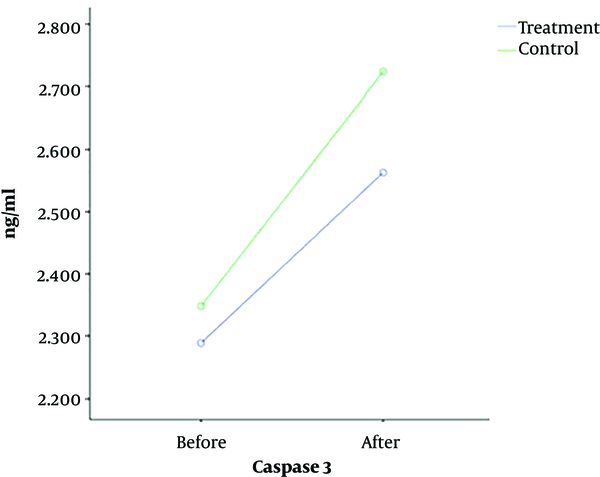
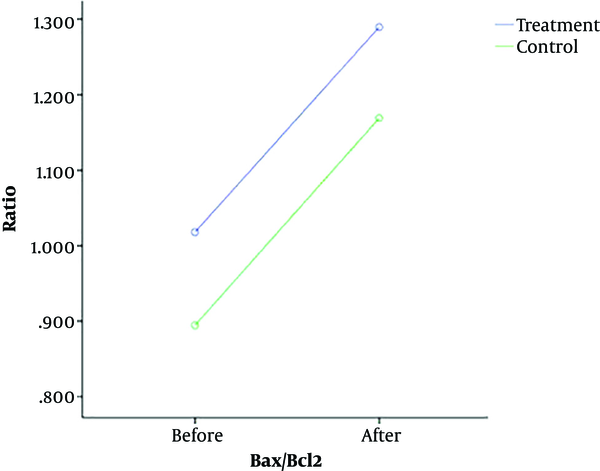
The caspase-3 levels in total, intervention, and control groups were 2.31 ± 0.33, 2.28 ± 0.35, and 2.34 ± 0.32 ng/mL before surgery and 2.64 ± 0.41, 2.56 ± 0.42, and 2.72 ± 0.39 ng/mL after surgery, respectively. In addition, the Bax/Bcl-2 levels in total, intervention, and control groups were 0.95 ± 0.09, 1.01 ± 0.11, and 0.89 ± 0.07 before surgery and 1.22 ± 0.16, 1.28 ± 0.14, and 1.16 ± 0.19 after surgery, respectively (Table 2).
| Apoptotic Factors | Groups | P-Value (Repeated Measures Test) | ||
|---|---|---|---|---|
| Intervention | Control | Total | ||
| Caspase-3 (ng/mL) | 0.11 | |||
| Before | 2.28 ± 0.35 | 2.34 ± 0.32 | 2.31 ± 33 | |
| After | 2.56 ± 0.42 | 2.72 ± 0.39 | 2.64 ± 0.41 | |
| Bax/Bcl-2 (ratio) | 0.95 | |||
| Before | 1.01 ± 0.11 | 0.89 ± 0.07 | 0.95 ± 0.09 | |
| After | 1.28 ± 0.14 | 1.16 ± 0.19 | 1.22 ± 0.16 | |
Apoptotic Factors Before and After Disc Herniation Surgery
4.4. Repeated Measures Test
Based on a repeated measures test, the CSF levels of caspase-3 (P = 0.11) and Bax/Bcl-2 (P = 0.95) were not significantly different between the intervention and control groups before and after surgery (Figures 2 and 3).
5. Discussion
We observed that the levels of two key apoptotic factors, namely Bax/Bcl-2 and caspase-3, significantly increased in disc herniation surgery, but dexmedetomidine, as an anxiolytic, sedative, and analgesic medication did not have any significant effect on CSF apoptotic factors’ levels in our study.
This investigation evaluated the intrathecal dexmedetomidine effect on apoptotic factors in the CSF. Jiang et al. in an observational study on patients with traumatic brain injury (TBI), measured apoptotic factors (caspase-3, sFas, caspase-9, and cytochrome c) in the CSF. They observed that these factors were significantly increased in patients who suffered from TBI (10). Mao et al., in different anesthesia in discectomy, observed that combined spinal-epidural anesthesia and epidural anesthesia caused no significant differences in immune function or inflammatory indices in patients with LDH (MAO) (11). In some other studies, there was a correlation between caspases 1, 3, 6, 7, 8, 9, and 12 and TBI (12, 13). In addition, in some studies on other apoptotic factors and TBI, there was a significant enhancement in CSF apoptotic factors (14, 15). Korshunova et al. observed that Bcl-2 could regulate apoptotic factors in myocardial ischemia/reperfusion injury (16). However, these studies were different from our study in terms of protocols. The increased levels of caspase-3 and Bax/Bcl-2 in the CSF of patients shortly after surgery led us to speculate that the surgical intervention may be the sole reason for this increase.
In some studies, the levels of apoptotic factors in brain tissue were measured in patients with cerebral stroke. These studies showed the pathway activation of caspases 1, 3, 8, 9, and 11, following cerebral ischemia (17); however, Askalan et al. suggested that cell death in infarcts following brain ischemia is partially caspase-3-independent and may also be attributed to nitric oxide (18). Alessandri et al. mentioned that caspase-dependent pathways may also occur in an acute subdural hematoma (19). Also, in a study by Montaner et al., they observed that the serum levels of the d-dimer and caspase-3 combination might be a biochemical marker for faster stroke diagnosis (20). Moreover, in prognosis evaluation, a study by Wang et al. mentioned that CSF caspase-3 enhancement at admission and three days after admission in aneurysmal SAH patients resulted in an unfavorable outcome. In addition, they observed that the levels of these factors were highly associated with injury severity and patients’ prognosis after SAH (21). On the same subject, Lorente et al. have recently reported that enhanced serum levels of caspase-3 are associated with increased mortality in severe TBI cases (22). Accordingly, most studies in this field have indicated that these factors could be prognostic in patients with different neurologic disorders.
5.1. Conclusion
We observed that dexmedetomidine as an anxiolytic, sedative, and analgesic medication in anesthesiology, if injected intrathecally, can reduce the NRS in patients undergoing lumbar open discectomy without any significant hemodynamic changes or other side effects, such as excessive sedation. This finding further confirms the earlier investigation results on this subject. Therefore, we recommend intrathecal dexmedetomidine injection for patients undergoing open discectomy as an adjunctive medication for postoperative pain control. Nevertheless, this medication did not significantly affect the CSF levels of apoptotic factors after discectomy. Finally, we believe that assessing the CSF levels of caspase-3 and Bax/Bcl-2 in a more extended postoperative period (i.e., eight, 24, and 48 hours) deserves further investigation.