1. Background
Migraine is one of the most common debilitating neurological disorders (1, 2). It is known as the recurrent headaches lasting for 4-72 hours. Headaches are usually felt in half of the head and are pulsating in nature. A progressive increase in migraine headaches can yield almost permanent pain that may last for at least 15 days per month. This condition is known as the transformed or chronic migraine (CM) (3). People with severe episodic migraine (EM) and CM have an extremely impaired quality of life, suffer from an immense disability, and impose a large financial burden on the country (1, 4). Up to 1/3 of migraine patients experience aura, which is a transient perceptual disturbance arising from the cortex (majorly visual cortex) or brainstem (5, 6).
The precise mechanisms underlying the pathophysiology of migraine are not well known yet. The complex interaction between hormones, neurotransmitters, and inflammatory pathways seems to drive the pathogenesis of migraine (7, 8). Traditional medications for migraine attacks are limited to analgesics (alone or in combination with caffeine), vasoconstrictors, and alpha adrenoreceptor antagonists such as ergotamine, and triptans (9). Some recent investigations focused on other therapeutic strategies such as modulation of the GABAA (γ-aminobutyric acid), serotonin (5-hydroxytryptamine), glutamate, CGRP (calcitonin gene-related peptide), and nNOS (neuronal nitric oxide synthesis) receptors for migraine treatment. These medications have been beneficial for both acute and preventive therapies (10-16).
Patients with CM are less responsive to these treatments (17, 18). It seems that to find new approaches in the treatment of CM, it is necessary to understand the molecular mechanisms involved in CM. Inappropriate treatment, overuse medication for acute migraine, obesity, chronic stress, and depression are the main risk factors for migraine transformation (19, 20). However, identifying the molecular factors that contribute to the change from EM to CM has received less attention and should be seen as a priority in migraine studies.
Transient receptor potential vanilloid 1 (TRPV1) that belongs to the TRP family of cation channels is permeable to Ca2+ and gated by capsaicin, harmful heat (> 43°C), protons, reactive oxygen species (ROS), and inflammatory agents (21-24). TRPV1 is an important mediator for peripheral and central sensitization and promotes hyperalgesia and allodynia. In this regard, TRPV1 is also likely to be involved in migraine pathophysiology (25, 26), importantly in pain generation and aura sensitization.
2. Objectives
The undefined pathways that trigger the transformation of EM to CM motivated us to investigate the alteration level of TRPV1 in the plasma, hippocampus, and somatosensory cortex of a rat model and compare these changes in EM and CM.
3. Methods
3.1. Animals
In this study, we used Wistar rats because of their anatomical, physiological, and genetic similarity to humans. A total of 18 healthy adult male Wistar rats with a weight range of 250 - 300 g were investigated. The animals were housed in the standard condition (12-h light/dark cycle, 22 ± 1°C, 45 - 50% humidity) and had free access to food and water. All experimental procedures were done in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Iran University of Medical Sciences and confirmed by the ethical committee of the National Institute for Medical Research Development (NIMAD) (ID: IR.NIMAD.REC.1396.054) (Grant no. 957537).
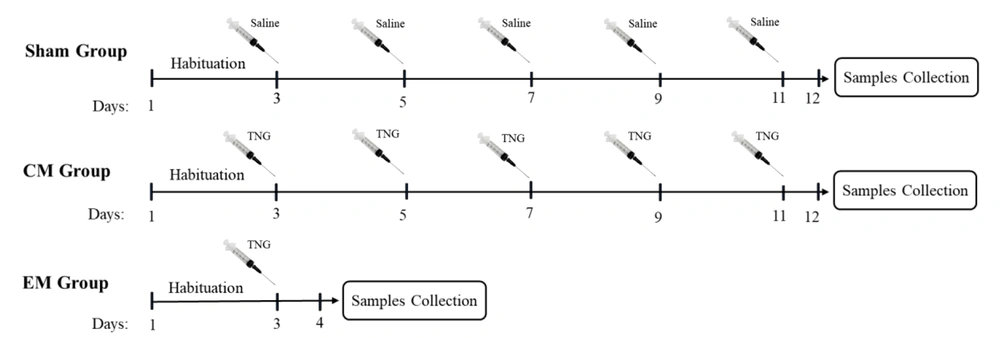
The animals were randomly assigned into three groups (n = 6 in each group) sham, episodic migraine (EM), and chronic migraine (CM). As shown in Figure 1, in the sham group, normal saline injections were administrated intraperitoneally (IP) every two days for 11 days. In the EM group, a single dose of trinitroglycerin (TNG) injection was administrated (IP; 10 mg/kg). In the CM group, injections of TNG were administrated every two days (on days 3, 5, 7, 9, and 11; IP; 10 mg/kg) (27). TNG was prepared by Pohl Boskamp Germany. All injections were done between 10:00 AM and 01:00 PM. After each injection, the animals were closely monitored by two examiners for symptoms of headache. Also, for more precise evaluation, the symptoms and behaviors were recorded by a video camera. To confirm pain induction after drug injection, Rat Grimace Scale (RGS) was used (28). This scale consists of five facial scales (orbital tightening, nose flattening, cheek flattening, ear changes, and whisker change) evaluating the rat behaviors after pain induction. In our study, after 5 minutes of TNG injection, signs of headache were observed in rats, which were similar to the clinical signs of migraine. These migraine signs peaked 30 minutes after the injection and lasted for 45 minutes. If an animal did not show headache symptoms after NTG injection, it was excluded from the study and replaced with another animal.
3.2. Plasma and Brain Tissue Gathering
After 24 hours of the last injection, the animals were anesthetized deeply with chloral hydrate (Sigma-Aldrich, 350 mg/kg, IP). Then, 2 mL of blood sample was drawn from the heart and collected in tubes coated with EDTA 7.5%. Samples were centrifuged for 15 minutes at 4000 rpm and 4°C. Next, their isolated plasma was stored at -80°C. Finally, the animal brains were removed manually, and the somatosensory cortex and hippocampus were dissected and stored at -80°C.
3.3. Assessments of TRPV1 Levels
The levels of TRPV1 in the plasma, as well as hippocampal and cortical tissues, were detected with an ELISA kit (Bioassay Technology Laboratory; E1568Ra) following the manufacturer’s instructions. The tissue samples were homogenized by adding 1 mL of PBS (PH 7.4) and then the supernatants were collected. A standard curve for the TRPV1 protein was constructed by plotting the mean absorbance (y-axis) versus the protein concentration (x-axis). According to the absorbance value of samples, the protein concentrations were calculated by linear regression of the standard curve.
3.4. Statistical Analysis
The data was presented as mean ± standard error. Statistical analysis was done using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Data analysis was performed using the SPSS software version 20. P-value < 0.05 was considered as significant.
4. Results
4.1. The Plasma Level of TRPV1
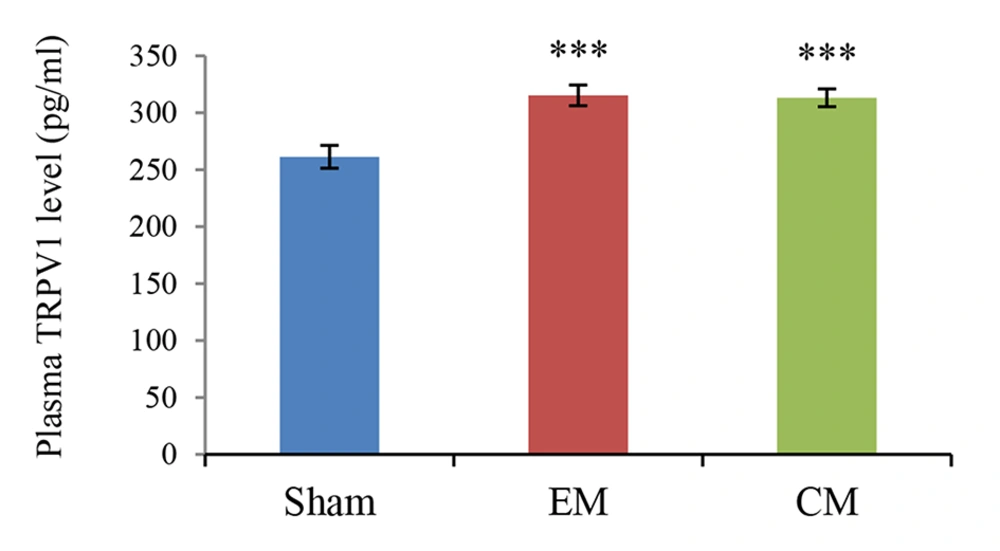
The mean plasma TRPV1 level (pg/mL) was presented in Figure 2. The plasma level of TRPV1 significantly increased in the EM and CM groups compared to the sham group (315 ± 9.04 & 313.14 ± 7.7 vs. 261.33 ± 10.1; P < 0.001 & P < 0.001 in both models).
4.2. Cortical and Hippocampal Level of TRPV1
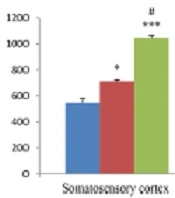
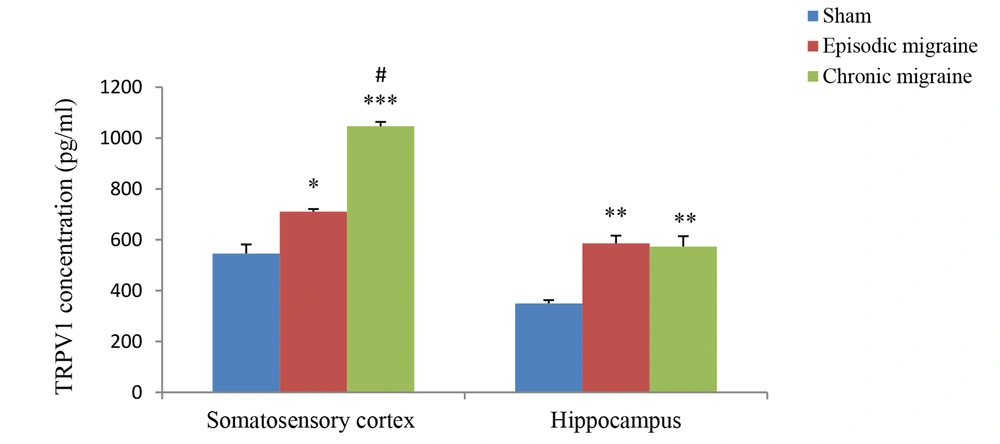
The results of hippocampal analysis showed that TRPV1 level (pg/mL) in this area increased in both EM and CM groups compared with the sham group (585.99 ± 30.11 and 573.27 ± 40.45 vs. 349.6 ± 12.98; P < 0.01 in both models), and the rate of this increase in both EM and CM groups was nearly the same. Also, the results of studying the somatosensory cortex displayed TRPV1 elevation in both EM and CM groups compared with the sham group (710.74 ± 10.2 and 1046.39 ± 17.25 vs. 545.73 ± 36; P < 0.05 & P < 0.001, respectively). Moreover, the post-test analysis revealed that TRPV1 enhancement in CM was much greater than in the EM, and a significant difference between the two conditions was observed (1046.39 ± 17.25 vs. 710.74 ± 10.2, P < 0.05) (Figure 3).
The bar graphs indicating the mean level of TRPV1 (pg/mL) in the cortex and hippocampus. There was a significant increase in the TRPV1 level in the cortex of CM group compared to the sham and EM groups. Also, there was a significant increase in the TRPV1 levels in the hippocampus of EM and CM groups compared to the sham group (** and *** P < 0.01 and P < 0.001, respectively vs. the sham group) (# P < 0.05 vs. the EM group).
5. Discussion
In this study, we investigated the alteration level of TRPV1 in the plasma, hippocampus, and somatosensory cortex of a rat model. The new aspect of this study was that the detection of the TRPV1 highlighted the EM to CM transformation.
Choosing the best animal model of a specific condition can result in valid and reliable findings that provide strong indications for human studies. In this study, we used the TNG model to carry out our research project. Today, TNG is known as the best animal model for migraine, which is highly similar to migraine patients in terms of clinical signs and symptoms (27).
Our findings showed that plasma TRPV1 level increased in EM and CM groups compared to the sham group, and the rate of this increase was nearly the same in both groups. Also, no significant difference was observed in the levels of TRPV1 in the CM group compared to the EM. Moreover, in the hippocampus, the same pattern of increase was observed in the TRPV1 in both EM and CM groups compared to the sham group; however, no remarkable difference was observed between the two migraine models. Interestingly, the results of the study in the somatosensory cortex showed that although in both EM and CM groups, the TRPV1 level increased compared to the sham group, this increase in the CM group was much greater than the EM, and a significant difference was observed between the two groups in this regard (P < 0.05).
According to the existing clinical evidence, an ongoing increase in headache attack frequency occurs in some patients with EM, which leads to CM. This state is often referred to as migraine transformation. Several risk factors for this progression are known, including age, socioeconomic status, genetic factors, obesity, barbiturates, opiates and caffeine overuse, stressful life events, and inflammation (19, 29). It is noteworthy that EM is transformed to CM more commonly in females than males (30).
In previous research, the molecular mechanism underlying migraine transformation has not been investigated efficiently. In a recent study, Yakubova et al. evaluated 46 patients suffering from migraines (27 EM and 19 CM) and demonstrated that TRPV1 single-nucleotide polymorphism (SNP) might be one of the effective factors for the progression of migraine from episodic to chronic form (31). Their results indicated that TRPV1 1911A>G SNP genotype is more frequent in CM patients than in patients with EM and healthy controls.
TRPV1 is broadly expressed in nociceptive fibers and has a key role in both pain perception and sensitization (32). Also, it has a wide distribution in the brain. It presents in the trigeminal nucleus caudalis, hypothalamus, thalamus, cerebral cortex, and several other brain regions (16, 33). TRPV1 activation and CGRP (calcitonin gene-related peptide) release in the trigeminovascular system causes neurogenic vasodilation, that has an important role in migraine development (25). The trigeminovascular system comprises of nociceptive neurons that arise from trigeminal ganglion and innervate the meninges, large cerebral arteries, and large venous sinuses. The projections from the trigeminal ganglion converge at the trigeminal nucleus caudalis. Fibers from the trigeminal nucleus caudalis projected to ventroposterior medial nucleus of the thalamus and from there projected to several cortical areas, including insular, auditory, visual, and olfactory cortices, as well as somatosensory cortex. These cortical pathways are responsible for the many cortically mediated specific symptoms of migraine (34, 35).
Previous studies have shown that somatosensory cortex is important for the consistency of migraine-induced changes. Several studies indicated structural and functional alteration of the somatosensory cortex in migraine. These findings clearly demonstrate that thickening of the somatosensory cortex is associated with increased duration and frequency of headaches (36-38). Also, a magnetoencephalographic study showed hyperexcitability of the primary somatosensory cortex in migraine, which is associated with the frequency of migraine attacks (39). Brain imaging investigations have reported a direct relationship between cerebral blood flow rate and the frequency of migraine attacks. Migraine patients are reported to display bilateral hyper-perfusion in the primary somatosensory cortex, where the value of blood flow is directly correlated to migraine attack frequency (40). To the best of our knowledge, this is the first study to provide evidence that TRPV1 levels are remarkably elevated in the somatosensory cortex in both EM and CM types. Moreover, our findings showed a significant elevation of somatosensory TRPV1 level in the CM compared to the EM and healthy controls. These results suggest that headache frequency might enhance through upregulation of somatosensory cortex TRPV1.
Activation of TRPV1 receptors stimulates CGRP release, which is one of the main mediators of migraine and gives long-lasting activation of meningeal afferents (31, 41). Plasma CGRP levels are increased during a migraine attack (42, 43) and also in the pain-free interval in CM; so, this peptide is considered as a CM biomarker (44, 45). The somatosensory function of CGRP has been implicated in the development of pain generation and neuronal sensitization in migraine (46).
Given that migraine attacks are repeated stressors and considering the key role of the hippocampus in stress response, functional and structural alterations of the hippocampus might be involved in migraine pathophysiology. Recently, it was shown that the expression level of TRPV1 both in the cortex and hippocampus increased in EM (47). In the present study, we examined the hippocampal TRPV1 alteration in both EM and CM types. The results of our study showed that after migraine induction, the amount of hippocampal TRPV1 increased in both EM and CM compared to the non-migraine rats. However, comparing the rate of these changes between the EM and CM groups showed that the rate of this increase was the same in both conditions. Therefore, although hippocampal TRPV1 can be involved in migraine development, TRPV1 changes in this region do not appear to play any role in migraine progression. However, our knowledge about TRPV1 alterations during migraine attacks is still inadequate. Mechanisms leading to increased TRPV1 expression in EM and CM are unclear, and further research in this area is needed.
In conclusion, we evaluated the TRPV1 level alteration in the plasma, hippocampus, and somatosensory cortex in the EM and CM groups using the rat model of migraine to investigate the possible role of TRPV1 levels in migraine progression. Our findings showed significantly higher elevation of TRPV1 in the CM group than the EM group in the somatosensory cortex. We discussed that further elevation of TRPV1 in the somatosensory cortex of rat models of CM compared to EM may indicate that this factor might contribute to increasing the severity and frequency of headache attacks in migraine and the progression from EM to CM. In this regard, paying attention to TRPV1 regulation in different areas of the brain in migraine patients is a new approach that could lead to the development of a new class of anti-migraine drugs that are useful in preventing the occurrence or progression of migraines.