1. Background
Spinal cord injury (SCI) is a serious neurological disease leading to poor quality of life due to serious both motor and sensory dysfunction, urinary bladder dysfunction, sexual dysfunction, and respiratory distress (1). It is estimated that 760,000 new cases of traumatic SCI occur annually due to falls, motor-vehicle crashes, traffic accidents, sports-associated accidents, and other causes (2). The medical treatments, rehabilitation training, hospitalization, and lifelong nursing, put a remarkable cost on both family and society (3). Although there are conventional methods, such as hemodynamic therapy, surgical decompression (4), corticosteroid administration (5), and spinal cord pressure monitoring (6) as well as novel strategies, including tissue engineering (TE) for treatment of SCI, these methods have been not completely succeeded to restore the injured spinal cord function (7-9). Therefore, it is urgent to find a new approach for the treatment of SCI.
Stem cell-based therapy, especially mesenchymal stem cell (MSC) transplantation, has been reported to be an effective therapeutic approach for traumatic SCI (9). However, several limitations, such as low survival rates and cell dedifferentiation, remain unresolved (10). TE or a combination of various biomaterials with stem cell transplantation has been recently reported in the scope of SCI (11, 12). Multifunctional scaffolds can carry the transplanted cells and therapeutic molecules in neural tissue engineering (NTE) (13). The combination of cellular and molecular therapies and scaffold materials has to be considered to overcome the challenges faced in central nervous system (CNS) tissue regeneration (10, 14).
ECM-based scaffolds, such as proteoglycans, glycoproteins, fibrin, collagen, and elastin, have been shown to be the best strategy for the development of materials suitable for tissue regeneration (TR) because they exactly mimic the mechanical and biological properties of the cell microenvironment seen experimentally. In native tissue, the cells reside in the extracellular matrixes (ECM), which are the complex fibrillary network composed of collagen, glycoproteins, elastin, proteoglycans, and various growth factors and cytokines. ECM creates a suitable structural framework to maintain tissue integrity. It also provides spatial and biochemical information for the cells that is critical for the regulation of cell behavior and intracellular signaling (15). Injectable hydrogels composed of collagen, fibrin, and other natural polymers have been shown to serve as scaffolds in TE (16-19). Since the ECM-based hydrogel scaffolds have been shown to be suitable for spinal cord TE, in this study, the regenerative effect of MSCs encapsulated into fibrin and collagen hydrogels scaffolds on rat model of SCI.
2. Objectives
The regenerative effect of adipose-derived AD-MSCs encapsulated into fibrin, and collagen hydrogel scaffolds on a rat model of SCI was investigated using clinical and histopathological examinations.
3. Methods
3.1. Characterization of the Isolated AD-MSCs
The AD-MSCs were isolated from peri-epididymal adipose tissue as previously described by Ebrahimi-Barough et al. (9). In brief, the adipose tissues were dissolved in 2 mg/mL of collagenase type I (Sigma-Aldrich) at 37°C for 1 hour and were gently shacked for 20 seconds at an interval of 15 minutes. To neutralize the collaenase, the suspension was treated with DMEM (Invitrogen) containing penicillin/streptomycin and 10% fetal bovine serum (FBS), filtered via 40- and 70-μm filters, and then centrifuged at 1500 rpm for 5 minutes. The cell pellet was suspended in DMEM media containing FBS (10%) and penicillin/streptomycin (1%). The media were changed at a four-day interval. AD-MSCs passage three were used for characterization.
3.1.1. AD-MSCs Characterization
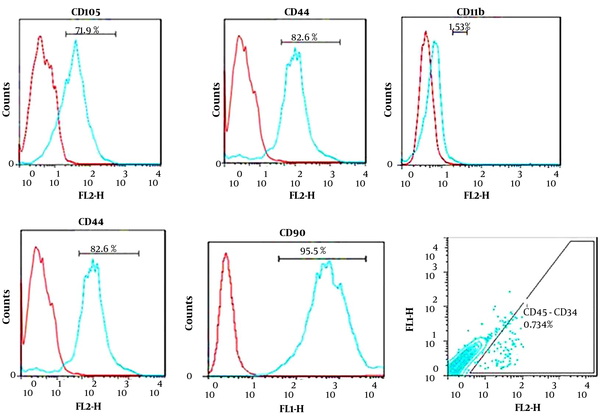
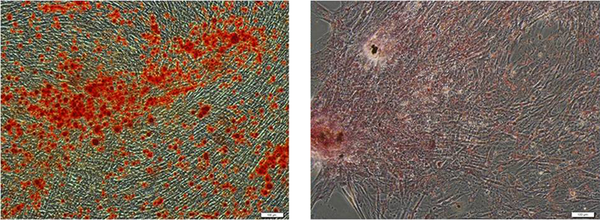
The isolated AD-MSCs were characterized using flow cytometry for the expression of specific surface markers, CD105, CD90, and CD73, and hematopoietic markers, such as CD11b, CD45, and CD34. The potential capacity of the isolated cells was assayed using differentiation of the isolated cells to osteoblast and lipobasts cells and stained using Alizarin Red S and Oil-Red-O, respectively.
3.2. Collagen and Fibrin Hydrogel Synthesis
Collagen was extracted from the rat tail as previously described (20, 21). A total of 1 g collagen was dissolved in 1 L acetic acid (0.001 M) for the preparation of the collagen scaffold. Then, 0.5 mL of the collagen solution was transferred to a 6-well plate, and 100 µL PBS (pH = 7.4) and 200 µL of DMEM were then added to each well. The plates were placed on ice to prevent premature gelation. To form a hydrogel, the plates were incubated at 37°C for 30 minutes.
A total of 1.5 mg of lyophilized bovine fibrinogen (Sigma, MO) was dissolved in 0.5 mL 199 M solution (Sigma Aldrich, MO) for preparation of the fibrin hydrogel. The solution was transferred to a 6-well culture dish. Then, 15μL of a thrombin solution (126 U/2.4 mL in 1 M NaCl; Sigma-Aldrich), 200 µL of DMEM, and 100 µL of FBS were added to the prepared solution, and the plate was incubated at 37°C for 3 hours to form the clot. Collagen and fibrin were then mixed with AD-MSCs suspension.
3.3. Animal Study
All in vivo steps of this study were done in accordance with the institutional guidelines of TUMS for animal care and use. Eighteen adults male Wistar rats (250 - 300 g) from a breeding colony were purchased and randomly divided into three equal groups, each with six rats, including the control or SCI group (SCI contusion model without treatment), SCI contusion model treated with AD-MSCs encapsulated in fibrin hydrogel, and spinal cord contusion treated with AD-MSCs encapsulated in collagen hydrogel groups. The rats were kept in individual cages in a 12-h light/dark cycle condition with free access to commercial food and tap water. SCI was induced using Precision Systems and Instrumentation, Lexington, according to the weight compression method (22). In brief, the rats were deeply anesthetized by intraperitoneal (IP) injection of ketamine and xylazine (80 and 10 mg/kg, respectively), and the dorsal skin was disinfected after shaving by ethanol 70% (v/v). The animals were then subjected to laminectomy at T9 and T10 (spinal T9-10) levels when the spinal column was exposed by a midline incision in this area. A 35-g weight with a concave shape and area of 6.6 mm2 was used to provide the equal compress and pressure distribution on the spinal cord for 15 minutes. One week after injury, the encapsulated AD-SCs (105) within both hydrogels were transplanted into lesion sites using a Hamilton syringe, and the muscles and skin were sutured. To hydrate the rats, 1 mL of lactated Ringer solutions was daily injected (IP) for five days post-SCI induction. The urinary bladders were expressed manually twice daily for two weeks post-SCI induction.
BBB locomotor rating score was done at 1 and 3 days post-treatment and weekly for four weeks for clinical assessment of recovery of motor function. SCI regeneration was also examined using immunohistochemical and histopathological analysis. The rats were sacrificed for microscopic analysis at week eight post-treatment.
3.4. Microscopic Examination
3.4.1. Histopathology
The spinal cord segments containing the lesion and graft were removed, fixed in 10% formalin buffer, embedded in paraffin, cut at 5-μm thickness, and stained using hematoxylin and eosin. The tissue sections were blindly examined by an expert pathologist, and the findings were recorded.
3.5. Statistical Analysis
Statistical analysis was conducted using SPSS version 22, and the data are expressed as the mean ± SEM. One-way ANOVA was used to analyze differences among all groups, followed by Tukey’s test. The P-values of less than 0.05 were considered significant.
4. Results
4.1. AD-MSCs Fabrication
The results of flow cytometry indicating specific-marker surface for MSCs and hematopoietic markers are shown in Figure 1.
These spindle shape isolated cells contained heterogeneous types of cells that mostly indicated the morphology of fibroblastic cells. These cells were differentiated into osteocytic and adipocytic cell lineages (Figure 2).
4.2. Clinical Assessment
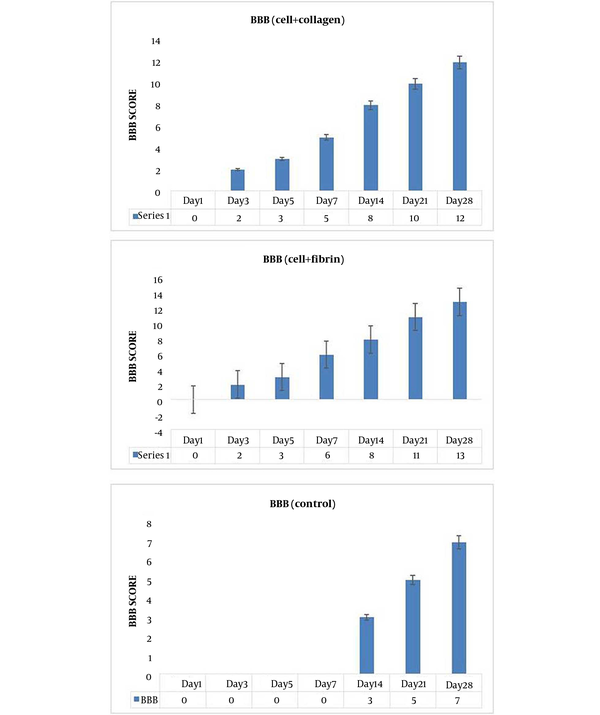
BBB scoring was used to evaluate the locomotion performance of SCI rats that is shown in Figure 1. After the treatment with both AD-MSC encapsulated with collagen and fibrin showed a gradual upward trend in the BBB score and faster functional recovery at all time points compared to the control group. On day four post-SCI induction, BBB score was assessed. For standardizing the severity and extent of the SCI, rats with hind limb score differences larger than four were excluded from the study. BBB score shows an improvement in hindlimb locomotion with collagen hydrogel + AD-MSCs and fibrin hydrogel + AD-MSCs from day three of post-implantation. The BBB scores of rats showed a gradual upward trend and an improvement in hindlimb in the treatment groups at all time points. However, treatment with collagen hydrogel + AD-MSCs led to a faster functional recovery than that of the control group. In the collagen hydrogel + AD-MSCs and collagen hydrogel + AD-MSCs groups, functional motor recovery significantly improved compared to the PBS group on days 3, 5, 7, 14, and 28 post-treatment (P < 0.05) (Figure 3).
4.3. Histopathological Findings
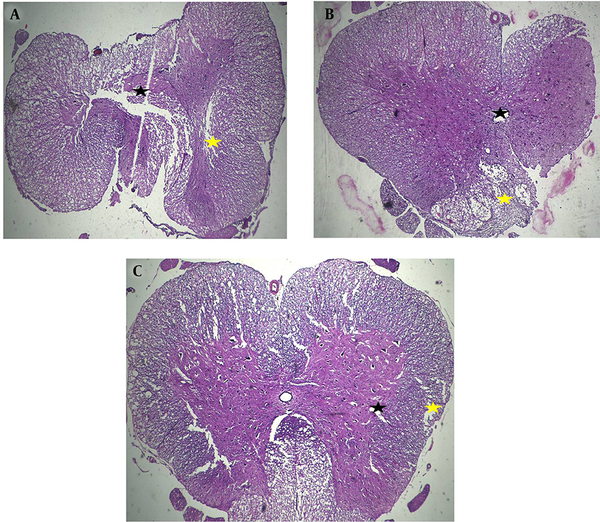
Severe polio and leuko-myelomalacia associated with disruption of spinal cord structure in the control group were identified. Also, mild polio and leuko-myelomalacia associated with mild to moderate disruption of spinal cord structure were seen in the fibrin hydrogel + AD-MSCs and collagen hydrogel + AD-MSCs groups (Figure 4). Compared to other groups, collagen seeded with stem cells could promote the nerve fibers regeneration and prevent vacuolization spaces in a rat model of SCI.
Hematoxylin and eosin staining was used for evaluation of treatment efficacy for filling the cavities of the injury site in each group in different experimental conditions, including the control (A) and AD-MSCs encapsulated into collagen (B) and fibrin (C) scaffold. Severe polio (black arrows) and leuko-myelomalacia (yellow arrows) associated with disruption of spinal cord structure in the control group was identified. Mild polio (black arrows) and leuko-myelomalacia (yellow arrows) associated with mild to moderate disruption of spinal cord structure was seen in both treatment groups, X40.
5. Discussion
The regenerative effect of MSCs encapsulated into ECM-based hydrogel scaffolds, fibrin, and collagen, on a rat model of SCI was investigated using clinical and histopathological evaluation in the present study. Functional recovery of injured tissue in the adult CNS disorders, such as SCI, and eliminating the surrounding inhibitory environment, remains a big challenge for clinicians. A total of 760,000 new cases of SCI occur due to falls, traffic accidents, and other causes annually in the world (2). Improvement of the rats with SCI using AD-MSC encapsulated with collagen and fibrin clinically and histopathologically was the main finding of the present study. Functional recovery or hindlimb locomotor activity was assessed using BBB scoring system, and faster functional recovery was seen in both treatment groups compared to the control group. However, there was no significant difference between fibrin and collagen scaffolds in terms of BBB score. BBB score is used to find the improvement of functional recovery and hindlimb locomotor activity in rats following SCI (23). The histological features of the spinal cord of the treatment were confirmed and were in accordance with the clinical features.
In the present study, fibrin and collagen hydrogels and MSCs were selected as the scaffold and cell sources, respectively, for SCI regeneration. Proper cells and scaffolds are two critical factors in regeneration medicine, such as neural regeneration medicine (8).
Several scaffolds have been introduced in NTE that are able to carry cells and therapeutic molecules (13). In the past years, studies have demonstrated that ECM-based scaffolds associated with MSCs played important roles in the treatment and regeneration of several organ disorders, such as SCI (15). Fibrin and collagen hydrogels have been attracted increasing attention for regeneration medicine, such as neural regeneration (10) due to high biocompatibility, ECM proteins, the binding sites of many cells, and growth factors (24). It has been reported that hydrogel is capable of axonal preservation and scarring events as well as modulating inflammation in a rodent model, resulting in improved hindlimb locomotor activity and functional recovery following SCI (12, 25). The excellent polymerization in situ at the injury site, the high capacity to imitate the natural ECM, the easy modification of physicochemical property, and acquiring a structure better matching the stump have made hydrogels in favor of the application to NTE to enhance the nerve regeneration for SCI (26). Collagen-based scaffolds have revolutionized the clinical treatment of severe injuries in peripheral nerves. Neural SCs deliveries through collagen scaffold have been shown to promote neuronal differentiation and locomotion recovery in SCI (27).
AD-MSCs were used as the source of cell implantation in the current study. A homogenous population of isolated stem cells was produced and grew in a fibroblast-like morphology during the third passage. The obtained data from flow cytometry analysis showed that the MSCs specific surface markers were expressed for the isolated cells while hematopoietic markers were expressed extremely low that was according to our previous study (28, 29). MSCs have been proven to have the potential to differentiate into glial cells and neurons (9, 30). MSCs can contribute to the regeneration of SCI at different phases. The motor function can be improved by the expression of several neurotrophic factors and cytokines, the regulation of the immune system, and the anti-apoptosis effect during the differentiation of the MSCs (31-33).
5.1. Conclusions
AD-MSCs encapsulated into fibrin and collagen hydrogels, as two of the most promising ECM-based or natural scaffolds have the potential to be developed in NTE for the treatment of SCI.