1. Introduction
Myasthenia gravis disease (MGD), Lambert-Eton myasthenic syndrome (LEMS), and inflammatory myopathy (IM) are three distinct autoimmune disorders that occur in primary or tumor-associated variants (1). The coexistence of MGD and IM is commonly reported in the literature and usually appears with thymic pathology (2, 3). Lambert-Eton myasthenic syndrome, an isolated variant of MGD, is rarely reported with IM (1, 4-8). The whole three disorders are autoimmune in nature and can be associated with malignancies such as breast, colon, and ovarian cancers and Non-Hodgkin lymphomas (1).
Lambert-Eton myasthenic syndrome is an autoimmune disease of the neuromuscular junction characterized by muscle weakness, autonomic dysfunction, and electromyographic findings of decrement response at low-frequency stimulation (3 Hz) of more than 10% and increment response after high-frequency stimulation (above 20 Hz) of more than 100% (9). Approximately 60% of patients with LEMS are paraneoplastic and associated with small cell carcinoma of the lung, detected within two years after the diagnosis (9). The other 40% is non-paraneoplastic, and no mechanism has declared the underlying cause. Because there is no documented serological marker for the paraneoplastic etiology, its association with cancers is difficult to establish. Voltage-gated calcium chancel (VGCC) receptor antibody is the most dominant marker in most LEMS patients. However, this antibody does not differ between the paraneoplastic and non-paraneoplastic variants of LEMS (10). Therefore, whole body computerized tomography (CT) scan, including brain, breast, chest, and abdomen, should be done to rule out any associated malignancy. Whole-body positron emission tomography with fluorodeoxyglucose (FDG-PET) should also be done for whom conventional imaging failed to detect metastatic tumors.
The association of LEMS with IM is extremely rare. To our knowledge, seven cases in the literature have been reported since 1984 (1, 4-8). Dermatomyositis and polymyositis (DM/PM) with T-cell inflammation were the most common IM-associated variants.
We report a 42-year-old, previously healthy female patient presenting with proximal muscle weakness of the four limbs, normal creatinine kinase (CK) level, and positive acetylcholine and voltage-gated calcium channel receptor antibodies. There were no oculobulbar symptoms and no history of thymoma, and the electrophysiological tests were non-specific. The whole-body CT scan did not declare any associated tumors. Muscle biopsy revealed focal perimysial and perivascular inflammation, predominantly B-cell, in non-necrotizing non-atrophic muscle fibers. The patient was diagnosed with LEMS associated with predominant B-cell inflammatory myopathy without malignancy. In this report, we describe this rare association and review the literature.
2. Case Presentation
A 41-year-old female, previously healthy, was referred to King Abdulaziz University Hospital in early 2022 for treatment with intravenous immunoglobulin (IVIG) after being diagnosed with Guillain-Barre syndrome based on a nerve conduction study (NCS). She was re-evaluated clinically. Her history started two months before her current referral, with rapidly progressive muscle weakness predominantly affecting upper limbs more than lower limbs and proximal muscle groups more than distal muscle groups. There was also a history of dry mouth and chronic constipation. The ocular and bulbar symptoms were absent.
Upon physical examination, it was observed that the muscle power of the proximal upper limb muscle groups was 3/5, and that of distal upper limb muscle groups was 4-/5. Regarding lower limbs, the motor power of proximal muscle groups was 3/5, while distally, it was 4+/5. Deep tendon reflexes were absent all over. In the sensory examination, pinprick sensation was slightly impaired in the distal limbs. Weakness of neck flexors was also observed (muscle power: 3/5). Otherwise, neither higher brain functions nor cranial nerve impairments were detected.
Laboratory investigations revealed mild leukopenia 2.48 (103/µL) [normal range 4.5 - 11 (103/µL)] and neutropenia 0.62 (103/µL) [normal range 1.8 - 7.7 (103/µL)]. Renal and liver functions, along with electrolytes, were within normal limits. Creatine Kinase (CK) was 62 U/L [normal range 26 - 162 U/L]. Brain and cervical magnetic resonance imaging (MRI) showed multiple degenerative changes in the cervical and lumbar discs. However, a lumbar puncture was also done, and the cerebrospinal fluid (CSF) analysis showed negative oligoclonal bands but with a slight increase in immunoglobulin G (53.1 [normal range, < 34 mg/L]). Nerve conduction study showed diffusely low compound motor amplitudes with normal velocities, F-wave, and latencies. Electromyography (EMG) was unremarkable. No repetitive nerve stimulations were observed. The differential diagnoses included non-specific inflammatory myopathies, paraneoplastic-induced myopathies, and brachial-cervical inflammatory myopathy.
As part of malignancy screening and to rule out occult malignancy, a PET CT scan did not show any hypermetabolic state uptake in the body. Furthermore, chest, abdomen, and pelvis CT scans were unremarkable. A breast mammogram with an ultrasound of both sides revealed only multiple tiny fibroadenomas (BIRAD3). Based on the clinical and laboratory results, a muscle biopsy was recommended.
2.1. Muscle Biopsy Procedure
Around 1.5 cm piece of right biceps muscle tissue was biopsied and submitted to a neuromuscular lab on a moistened saline-Teflon gauze. According to our laboratory protocol, the freezing process immediately started using 2-methylbutane and -80°C liquid nitrogen. The frozen fragments were sectioned at 6 - 9 μm thicknesses in a cryostat machine at -30°C.
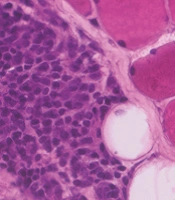
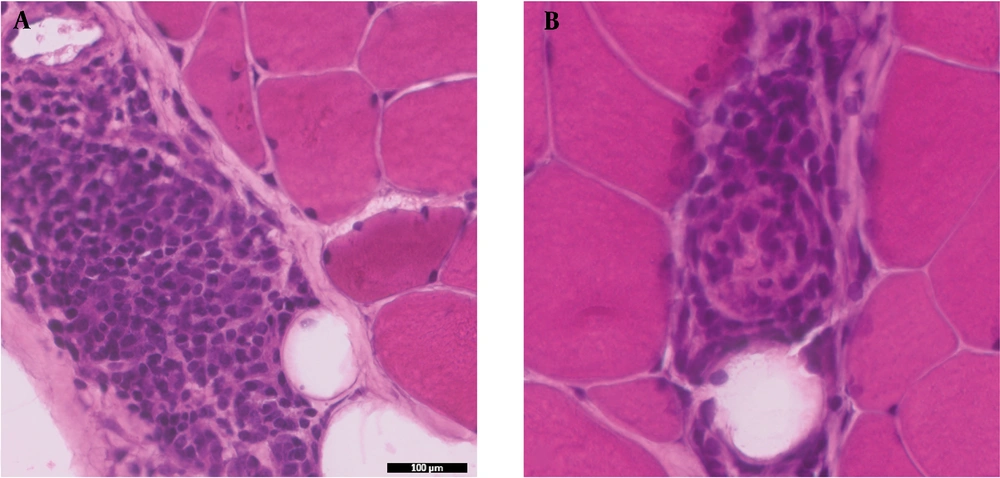
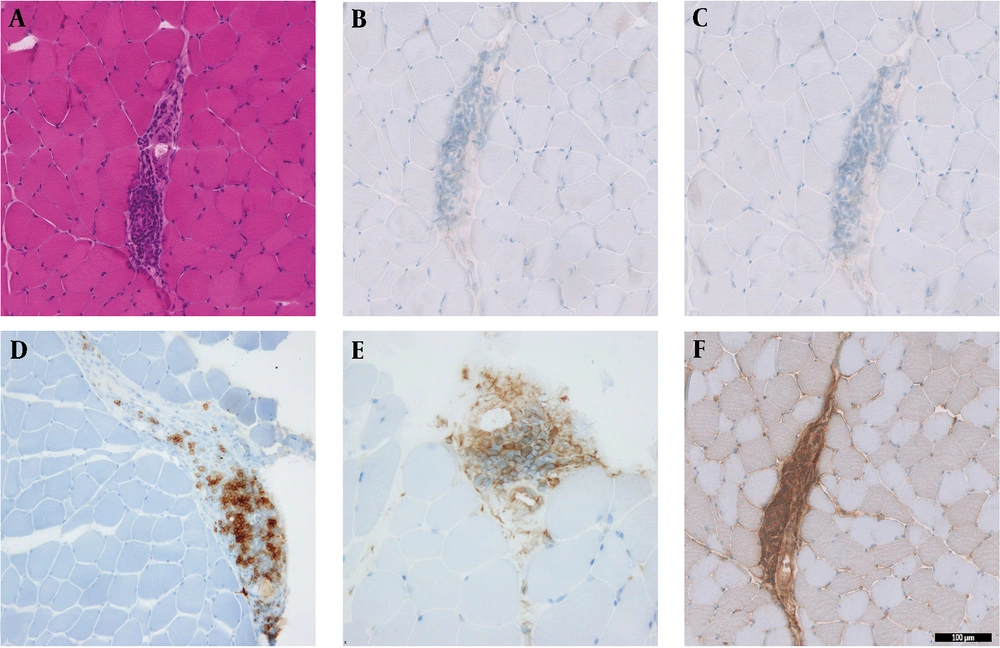
Hematoxylin and Eosin (H&E) stained sections showed minimal variability in muscle fiber size and shape. There was no evidence of muscle fiber atrophy, necrosis, or regeneration. There was no perifascicular pathology or perimysial fragmentation. The only pathological finding in this biopsy was focal areas of perimysial and perivascular inflammation (Figures 1A, B and 2A). The inflammation was mainly CD20+B-lymphocytic cell component, while there was no T-cell component (CD4- and CD8-) (Figure 2B-D). A few CD68+ macrophages were also identified (Figure 2E). Major histocompatibility (MHC) Class-I (Clone W6/32- Cat# Ab22432 from Abcam, France) was performed with an automated stainer using a DAB detection kit (Ventana, Tuscon, AZ, US). The MHC Class I stain labeled the endomysial capillaries and was overexpressed in the area where the perimysial/perivascular inflammation was present. There was no upregulation in muscle fibers (Figure 2F). The oxidative enzymatic stains (NADH, COX, and SDH) showed unremarkable results.
Histological sections from the biceps muscle show focal areas of perimysial and perivascular inflammation in non-necrotizing muscle fibers. A, H&E sections of inflammation; B, inflammation with CD4- T-cells; and C, CD8- T-cells; D, predominant CD20+ B-cells; E, CD68+ macrophages; F, MHC-class I expression in the focal inflammation (magnification 20X).
Serological tests for paraneoplastic antibodies, including anti-Hu and DNAse, were undetected (normal range of anti-Hu less than 1:50 titer) and 104 (normal range < 301 u/L), respectively. Acetylcholine receptor (AChR) antibodies were 0.66 nmol/L (normal range < 0.25 nmol/L), while VGCC receptor antibody was 58.1 pmol/L (normal range < 40 pmol/L).
Based on the clinicopathological correlation, the diagnosis was consistent with LEMS, with a predominant B-cell lymphocytic variant of inflammatory myopathy diagnosis. The patient started a LEMS treatment regimen, which included pyridostigmine bromide (60 mg tablet, QID), prednisolone (60 mg OD, tapered gradually to 10 mg OD), and azathioprine (50 mg BID). One month later, the patient showed tangible improvement in her symptoms, including muscle power and daily activity.
3. Discussion
Although IMs have been reported in association with MGD, their association with LEMS is extremely rare, and only seven cases were documented in the literature (Table 1) (1-8). The whole three autoimmune disorders (MGD, LEMS, and IMS) were proven to be associated with different types of malignancy (1).
| Authors | IMs Type | Inflammation Type | CK Level* | Malignancy | Treatment |
|---|---|---|---|---|---|
| Artigo et al. (4) | Dermatomyositis | T-cell | Unknown | Yes | Steroid |
| Mossner et al. (1) | Polymyositis | T-cell | 3191 | No | Steroid + diaminopyridine |
| Mossner et al. (1) | Polymyositis | T-cell | 1073 | No | Steroid + cyclosporin |
| Milanez et al. (5) | Dermatomyositis | T-cell | 889 | Lung cancer | None |
| Hill et al. (8) | Dermatomyositis | T-cell | 1178 | Breast cancer | IVIG + steroid |
| Dai et al. (6) | Dermatomyositis | T-cell | 436 | Lung cancer | None |
| Isfort et al. (7) | Dermatomyositis | T-cell | Unknown | No | Unknown |
| Kurdi (present study) | Non-PM/DM | B-cell | 62 | No | Steroid + azathioprine |
All Reported Cases of Lambert-Eaton Myasthenic Syndrome (LEMS) with Inflammatory Myopathies (IMs) in the Literature
Although 60% of patients with LEMS were paraneoplastic, only four reported cases were associated with IMs (DM/PM spectrum) (4-6, 8, 9). The non-paraneoplastic variant of LEMS-associated IMs is extremely rare, and the underlying mechanism is still unclear (7). Because no serological marker for the paraneoplastic variant of LEMS has been explored, the association with malignancy is currently difficult to establish. The VGCC receptor antibody does not differ between the paraneoplastic and non-paraneoplastic variants of LEMS (10). Our case was diagnosed as a non-paraneoplastic variant of LEMS, as there was no history of malignancy. The serological markers were also inconclusive.
The type of IM and the subtype of underlying inflammation are also various in the previously reported cases (Table 1) (1, 4-8). Most of the reported cases of LEMS were categorized as Dermatomyositis/polymyositis (DM/PM) spectrum, and T-cell inflammation was the most common predominant subtype (Table 1) (1, 4-7). Mossner et al. (1) reported two cases of PM associated with T-cell lymphocytic inflammation. These two cases were not associated with malignancy. The other two reported cases were associated with DM and lung cancer (5, 6). Our case showed a non-specific type of myositis (non-DM/PM spectrum). Some areas of inflammation predominantly had B-cell lymphocytic components with no T-cell component, and the MHC class I was upregulated (Figure 2).
This rare association of LEMS with predominant B-cell lymphocytic inflammation in non-diseased muscle fibers has never been reported in the literature. The mechanism of this association is not well-understood in our case. Generally, B-cell inflammation in MGD has been previously reported in cases of brachio-cervical inflammatory myopathy (BCIM) (11). However, it was observed that the most common clinical findings in these patients were proximal upper limb weakness along with neck muscles with the absence of lower limb involvement (11). Focal perimysial and perivascular inflammation with prominent B-cell component, C5b-9 staining of endomysium, and presence of serum ANAs are considered part of the myopathological findings of BCIM (11). Although predominant B-cell lymphocytic inflammation in non-necrotizing muscle fibers mimics the diagnosis of BCIM, our case showed a completely different clinical picture. The lower limbs were involved in our patient.
Both IMs and LEMS/MGD have underlying inflammatory and autoimmune mechanisms; thus, immunosuppressive medications can induce a response in such cases (2). Our case responded well to steroids and immunosuppressive therapy and showed good improvement within two weeks of the treatment.
3.1. Conclusions
We reported the first case of LEMS, unusually associated with predominant B-cells inflammatory myopathy in the absence of malignancy. Clinical investigations and muscle biopsies helped establish the diagnosis. This variant should not be misdiagnosed with BCIM. Because the mechanism is not well clarified, further research is needed in this area to understand underlying pathogenesis.