1. Background
Epilepsy is one of the brain disorders characterized by frequent and sudden seizures and affects half to one percent of the world population (1). In epilepsy, the excitability of neurons is permanently increased in the central nervous system (CNS) following an imbalance between the excitatory and inhibitory systems (2), thus causing functional alteration of ion channels and neurotransmitter receptors and increasing the permeability of the blood-brain barrier and expression changes of the glutamate receptor subunits (3, 4). In more than 70% of patients with epilepsy, seizures are suppressed by antiepileptic drugs (5). Some major side effects of antiepileptic drugs, such as neurotoxicity and hepatotoxicity, have attracted the attention of researchers to find non-medicinal alternatives (6, 7).
New studies show that sports interventions can improve cognitive functions, increase brain activity, and reduce psychological symptoms (8, 9). Both high- and moderate-intensity aerobic exercises increase the level of neurotrophins and neurotransmitters and improve psycho-cognitive performance at all ages (10, 11). Also, exercise can reduce the severity of seizures and delay their onset (2). One of the mechanisms through which exercise leads to the improvement of brain function is the potentiation of neurotrophic factors (NTFs). Neurotrophic factors are small proteins that have key roles in the regeneration of nerve cells and neural survival (12). Nerve growth factor (NGF) is essential for the growth and maintenance of the phenotype of neurons in the peripheral nervous system and the functional integrity of cholinergic neurons in the CNS (13). Also, glial cell-derived growth factor (GDNF) is critical for neuronal survival and neurogenesis (14).
Previous studies have reported exercise stimulates the regeneration of nerve cells by increasing the expression of NGF (15). Exercises also lead to the production of GDNF factor produced by the glial cells of the substantia nigra, where dopaminergic neurons are located. Therefore, exercise improves plasticity in dopaminergic neurons by increasing the production of GDNF (16). TRPV1 is one of the transient potential receptors (TRP) that belongs to the family of non-specific voltage-gated and ligand-gated ion channels and is more permeable to calcium than other cations. The expression level of TRPV1 in the CNS is significantly lower than in the peripheral system, but this small amount plays an important and significant effect on the central (17, 18). There is evidence that TRPV1 receptors in the brain are involved in many fundamental functions, including synaptic transmission and synaptic plasticity. TRPV1 expression is variable and depends on the presence or absence of growth factors. Considerable studies on TRPV1 receptors indicate that these receptors can be considered specific targets for antiepileptic compounds (19). Evidence suggests that NTFs influence seizure-related processes through the regulation of TRPV1 receptors (20).
Even though the nervous system is one of the parts that has always been of interest in adapting to exercises. There has not been enough research regarding clarifying the influence and possible compatibility of TRPV1 with sports training. But the role of this receptor is prominent in the goals related to the health of sports.
2. Objectives
This study aimed to investigate the effectiveness of exercise on neurotrophic factors by influencing the expression of vanilloid receptor type 1 (TRPV1). Considering the role of moderate physical exercise on seizure alleviation and also its role in the activation of neurotrophic factors, this study aimed to investigate whether TRPV1 modulation through neurotrophic factors activation in the hippocampus may be one of the underlying mechanisms for exercise effectiveness in epilepsy or not.
3. Methods
3.1. Animals
Thirty adult male Wistar-Bauzen rats weighing 250 to 300 g were placed in a controlled environment with a light-dark cycle at a temperature of 22 ± 1°C for one week before the beginning of the experiment. All experiments were performed according to the protocol approved by the ethical principles of working with laboratory animals of Iran University of Medical Sciences (IR.IUMS.REC.1399.023). Animals were randomly divided into five groups (n = 6). (1) Seizure group: Seizure was induced by intraperitoneal (IP) injection of Pentylenetetrazol (PTZ) every other day for four weeks. (2) Sham group: 0.9% normal saline was injected according to the PTZ protocol. (3) Exercise group (EX): The animals in this group had to run on a treadmill for 30 minutes five days a week in weeks one to four. (4) Exercise and seizure group (EX+PTZ): Seizure and exercise were performed for four weeks. Animals were forced to run on a treadmill five days a week, and seizures were performed five hours after exercise according to the seizure group protocol. (5) Exercise before seizure induction group (EX-PTZ): Animals in this group were forced to do exercise for four weeks like the exercise group (without PTZ injection). In weeks five to eight, seizure induction and exercise were done according to the protocol of the exercise and seizure group (EX + PTZ).
3.2. Treadmill Exercise Protocol
Rats in EX, EX+PTZ, and EX-PTZ groups were forced to run on a treadmill for 30 minutes, five days a week. The speed of the treadmill was gradually increased at a zero-degree incline (5 m/min every five min, up to 25 m/min) (2). The first and last three minutes were considered warm-up and cool-down, respectively.
3.3. Immunohistofluorescence
After the last injection and exercise, the animals were anesthetized with 350 mg/kg chloroform (Sigma–Aldrich). After complete anesthesia, heart perfusion was performed first with 250 mL of saline and then with 400 mL of paraformaldehyde (PFA) solution. After the completion of perfusion, the head of the animal was detached, and the brain was removed from the skull and placed in 4% paraformaldehyde at 4°C for one week (21).
Tissue samples were embedded in paraffin blocks and then coronally cut with a thickness of 8 µm by microtome (21). Three parts of each brain were selected and deparaffinized at a distance of 30 µm. Then they were dehydrated by xylene and alcohol and washed three times with phosphate-buffered saline (PBS). Then the slices were washed three times in Tris wash buffer (Three minutes each time) and with a secondary antibody (Goat polyclonal Anti Rabbit IgG) conjugated with DyLight 484 (Cat.no: GTX213110-04) for one hour at room temperature. The chamber was incubated and washed three more times in Tris wash buffer (for three minutes each). Samples were photographed for statistical analysis using a digital camera (Nikon, objective lens 40×) connected to a fluorescent microscope (100 Ts Nikon Florescent).
3.4. Statistical Analysis
All the values were presented using Mean ± standard error of mean (S.E.M). The data were analyzed by SPSS software. One-way ANONA analysis was used to compare the experimental groups and followed by post-hoc Tukey's test for between groups comparison. The significance level of the tests was considered P < 0.05.
4. Results
4.1. The Effect of Treadmill Exercise on the Distribution of NGF, GDNF, and TRPV1 Receptor
4.1.1. Distribution of NGF in Hippocampal Regions
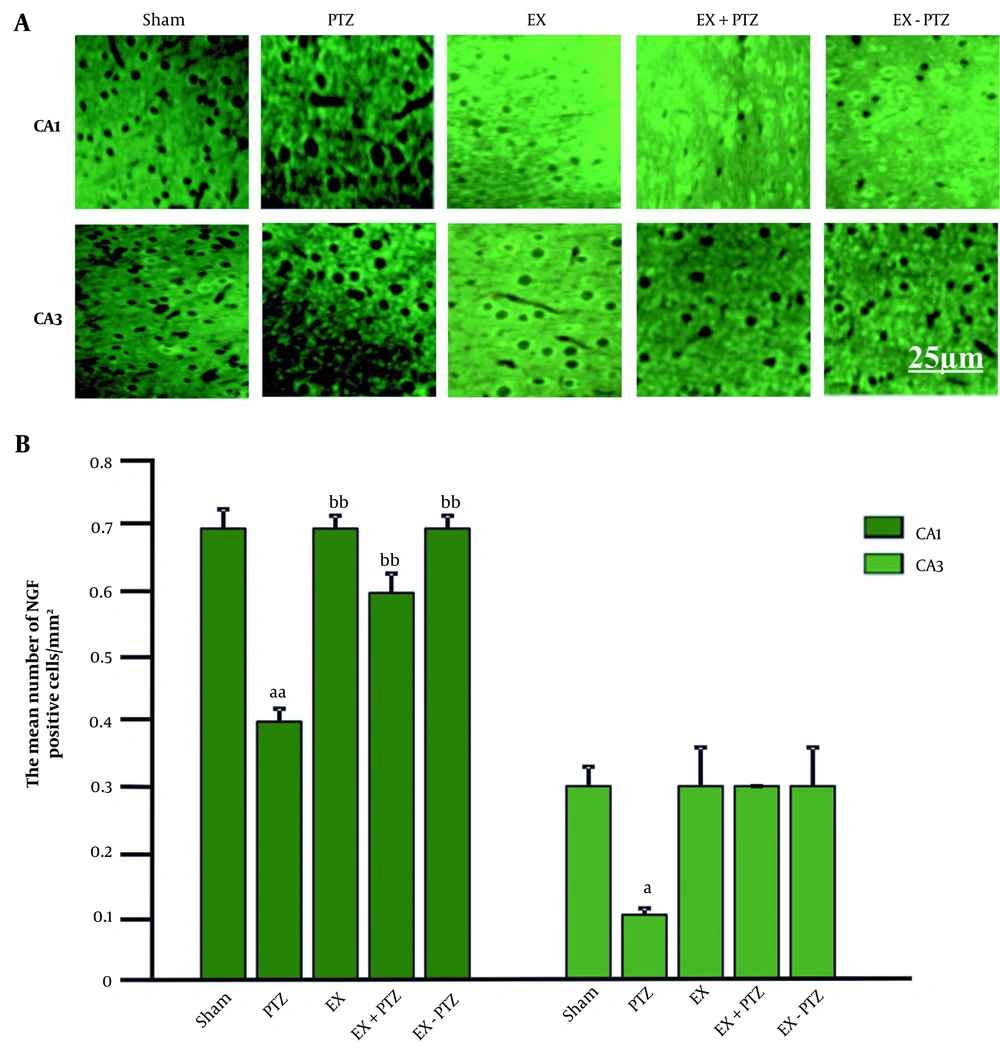
The mean percentage of NGF cells in the CA1 region in the PTZ group (0.4 ± 0.02) was significantly decreased compared to the Sham group (0.7 ± 0.03) (P < 0.01). There was no significant difference compared to the Sham group in EX+PTZ (0.6 ± 0.03), EX (0.7 ± 0.02), and EX-PTZ (0.7 ± 0.02) groups, (respectively, P = 0.4 and P = 0.9). Also, in the EX+PTZ group, EX and EX-PTZ showed a significant increase compared to the PTZ group (P < 0.01) (Figure 1B).
The mean percentage of NGF cells in the CA3 region in the PTZ group was (0.1 ± 0.01) compared to the Sham group (0.3 ± 0.03), which has decreased significantly (P < 0.05). However, in EX+PTZ (0.3 ± 0.0), EX (0.3 ± 0.06), and EX-PTZ (0.3 ± 0.06) groups, there was no significant difference compared to the Sham group (P = 0.6 and P = 1, respectively). Also, in the EX+PTZ, EX, and EX-PTZ groups, there was no significant difference compared to the PTZ group (P = 0.3 and P = 0.06, respectively). The distribution of NGF in the CA1 and CA3 regions in the PTZ group was significantly reduced compared to the Sham group. The distribution of NGF in the CA1 region showed a significant increase in the EX+PTZ, EX, and EX-PTZ groups compared to the PTZ group. In the bar chart, a and aa indicate P < 0.05 and P < 0.01 compared to the Sham group, respectively and bb indicates P < 0.01 compared to the PTZ group (Figure 1).
4.1.2. Distribution of GDNF in Hippocampal Regions
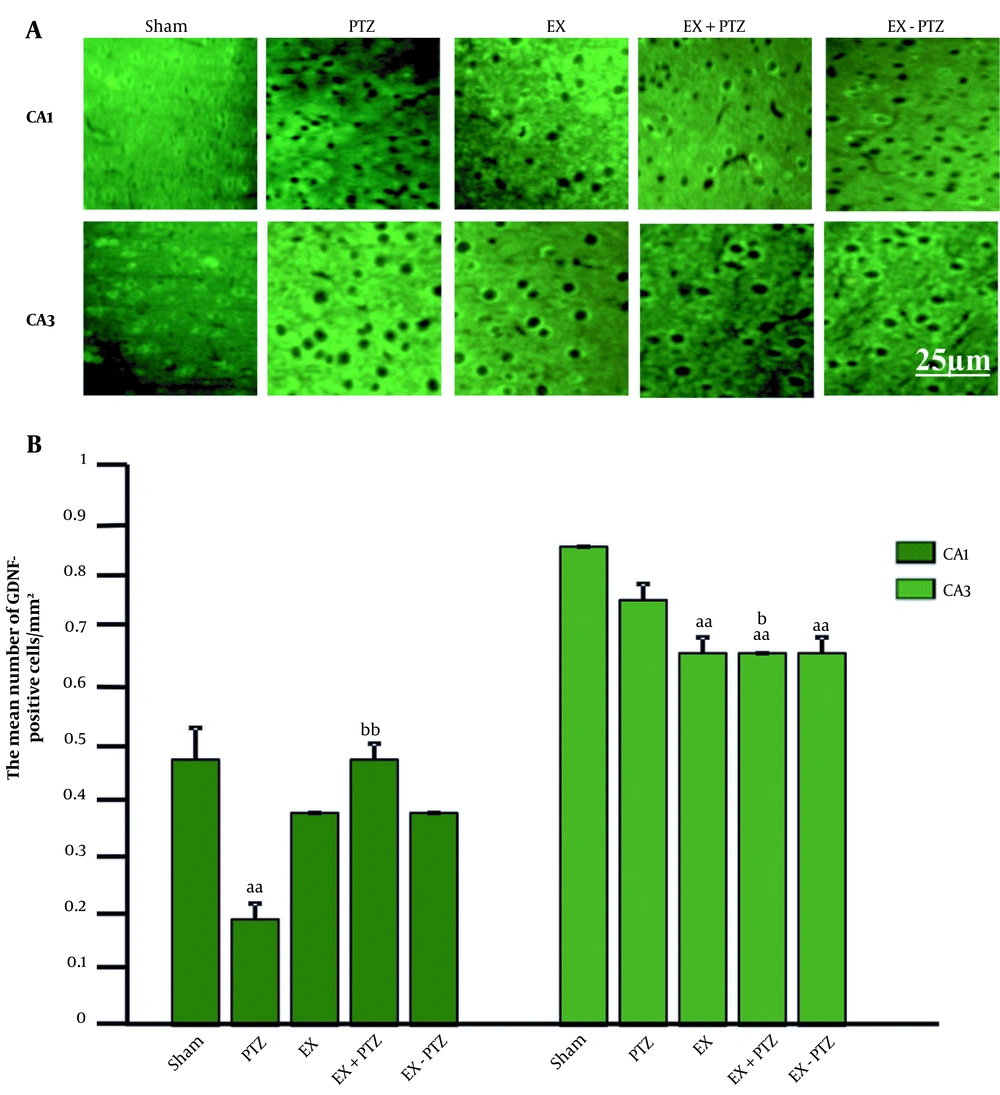
The mean ± standard deviation (SD) percentage of GDNF cells in the CA1 region in the PTZ group (0.2 ± 0.03) showed a significant decrease compared to the Sham group (0.5 ± 0.06) (P < 0.01). But in the EX+PTZ (0.5 ± 0.03), EX (0.4 ± 0.0), and EX-PTZ (0.4 ± 0.0) groups, it did not show any significant difference compared to the Sham group (P = 1 and P = 0.1, respectively). It also showed a significant increase in the EX+PTZ group compared to the PTZ group (P < 0.01). However, there was no significant difference in EX and EX-PTZ groups compared to the PTZ group (P = 0.2) (Figure 2B).
The mean ± SD percentage of GDNF cells in the CA3 region in the PTZ group (0.8 ± 0.03) compared to the Sham group (0.9 ± 0.0) did not show a significant difference (P = 0.3). Also, in the EX+PTZ (0.7 ± 0.0), EX (0.7 ± 0.03), and EX-PTZ (0.7 ± 0.03) groups, it showed a significant decrease compared to the Sham group (P < 0.01). In addition, it showed a significant decrease in the EX+PTZ group compared to the PTZ group (P < 0.05). However, there was no significant difference in EX and EX-PTZ groups compared to the PTZ group (P = 0.09).
The distribution of GDNF in the CA1 region In the PTZ group and the CA3 region in the EX+PTZ, EX, and EX-PTZ groups was significantly reduced compared to the Sham group. It showed a significant increase and decrease in the CA1 and CA3 regions in the EX+PTZ group compared to the PTZ group, respectively. In the bar chart, aa indicates P < 0.01 compared to the Sham group, and b and bb indicate P < 0.05 and P < 0.01 compared to the PTZ group, respectively.
4.1.3. Distribution of TRPV1 Receptor in Hippocampal Regions
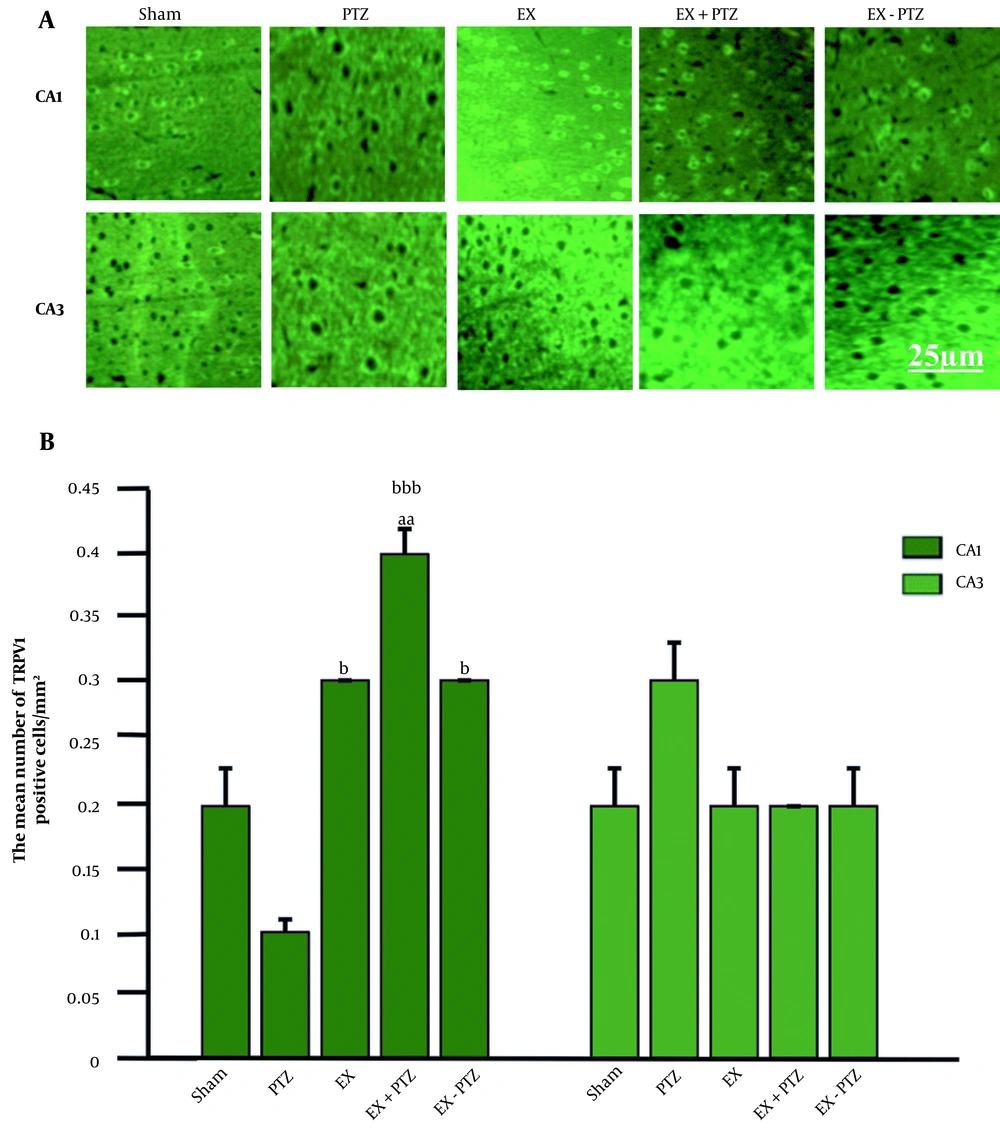
The mean ± SD percentage of TRPV1 receptor distribution in the CA1 region in the PTZ (0.1 ± 0.01), EX (0.3 ± 0.0), and EX-PTZ (0.3 ± 0.0) groups did not show any significant difference compared to the Sham group (0.2 ± 0.03) (P = 0.1 and P = 0.4, respectively). But in the EX+PTZ group (0.4 ± 0.02), it showed a significant increase compared to the Sham group (P < 0.01). Also, the mean percentage of TRPV1 receptor distribution in the EX+PTZ, EX, and EX-PTZ groups showed a significant increase compared to the PTZ group (P < 0.001 and P < 0.05, respectively) (Figure 3B).
TRPV1 distribution in hippocampal regions. (A) Immunofluorescent microscopic image of TRPV1 cells. (B) Bar chart of the effect of PTZ and exercise on TRPV1 distribution. aa indicates P < 0.01 compared to the Sham group, b and bbb indicate P < 0.05 and P < 0.001 compared to the PTZ group, respectively.
The mean ± SD percentage of TRPV1 receptor distribution in the CA3 region in PTZ (0.3 ± 0.03), EX+PTZ (0.2 ± 0.0), EX (0.2 ± 0.03), and EX-PTZ (0.2 ± 0.03) groups compared to Sham group (0.2 ± 0.03) showed no significant difference (P = 0.1 and P = 0.8, respectively). Also, in the EX+PTZ, EX, and EX-PTZ groups, there was no significant difference compared to the PTZ group (P = 0.06 and P = 0.4, respectively).
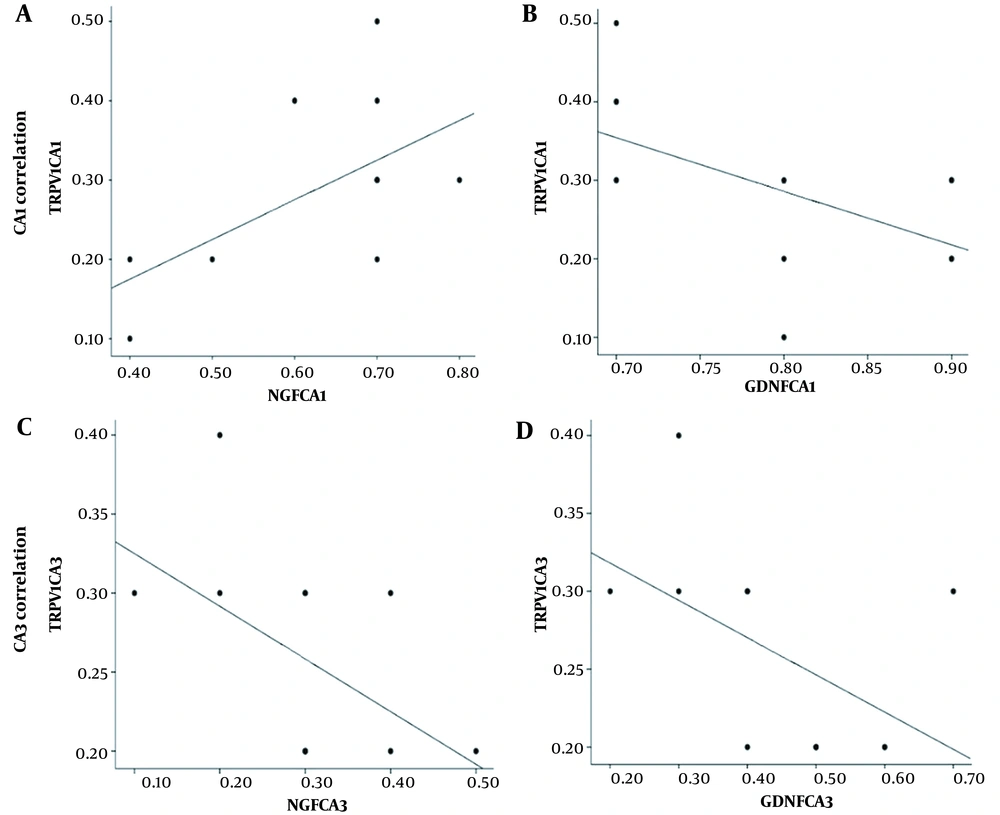
4.2. Correlation Between Expression of NGF/GDNF and TRPV1
The relationship between NGF/GDNF and TRPV1 expression in CA1 and CA3 regions is presented (Figure 4). The correlation between NGF and TRPV1 expression in the CA1 region showed that high NGF expression (Figure 4A; r = 0.36, P < 0.05) was associated with high TRPV1 level, but there was no significant correlation between GDNF and TRPV1 expression (Figure 4B; r = 0.32, P = 0.05). The correlation between the expression of NGF/GDNF and TRPV1 in the CA3 region showed that there was no significant correlation between the expression of NGF and TRPV1 (Figure 4C; r = 0.27, P = 0.08) and also between the expression of GDNF and TRPV1 (Figure 4D; r = 0.26, P = 0.08). (A) and B) scatter plots show the correlation between NGF/GDNF and TRPV1 expression in the CA1 region. A strong correlation between NGF and TRPV1 expression was shown in the CA1 region. (C) and (D) scatter plots show the correlation between NGF/GDNF and TRPV1 expression in the CA3 region. There was no significant correlation between the expression of NGF/GDNF and TRPV1 in the CA3 region.
The correlation between protein expression of NGF/GDNF and TRPV1. Scatter plots are shown in the CA1 region (A and B). There is a strong correlation between NGF and TRPV1 expression, but there was no significant correlation between the expression of NGF/GDNF and TRPV1 in the CA3 region (C and D, respectively).
5. Discussion
5.1. Regulating the Neurotrophic Factors by Doing Exercise in Epilepsy
Our results showed a significant increase in the expression of NGF in the CA1 region in the EX, EX+PTZ, and EX-PTZ groups, as well as an increase in the expression of GDNF in the EX+PTZ group compared to the PTZ group. Seizures are followed by excessive immune responses and imbalances in synaptic transmitters (22). Immune activation by increasing the production of pro-inflammatory cytokines increases the potential for new convulsions (23). After seizure induction, inflammatory mediators released from glial cells, including interleukin-1beta (IL-1β), IL-6, IL-10, and tumor necrosis factor-alpha (TNF-α), increase in the brain, which directly leads to activation of their pathways involved in apoptosis through their receptors in neurons (24). They stimulate convulsions by affecting ion channels, and neurotransmitter receptors and increasing the permeability of the blood-brain barrier (25). On the contrary, long-term and moderate-intensity exercise training (65% VO2 Max) causes a clear decrease in the level of inflammatory cytokines, including IL-1β and IL-6, in the hippocampus and cerebellum (26, 27), and by improving the inflammatory condition and decreasing oxidative stress (28). It increases the expression of neurotrophic factors in the hippocampus of mice and rats (29).
One of the mechanisms of exercise that leads to the improvement of brain function is neurotrophic factors, which play an important role in the survival of neurons that are affected by the processes of neuronal destruction. Neurotrophic factors increase the growth and function of damaged neurons and increase neuronal survival and neurogenesis by preventing nerve cell death (30). Several previous studies have reported that regular aerobic exercise is effective in enhancing neuroprotection by increasing NGF secretion and leading to NGF upregulation (15). Also, increasing NGF secretion has a direct relationship with memory improvement and an inverse relationship with oxidative stress (31). Four weeks of endurance exercise on a treadmill has been shown that is related to the reduction of the effects of age-related progressive neurodegenerative diseases such as Parkinson's by increasing striatal NGF levels (32). Studies have shown that moderate-intensity treadmill exercise increases the level of NGF and prevents the apoptosis of nerve cells in nerve-damaged mice, and reduces the death and demyelination of neurons activating P-PL3K (33). Aerobic exercise can play a significant role in improving learning, increasing GDNF expression, and reducing inflammation caused by glial cells (34). Also, GDNF can have an anti-inflammatory and anti-neurotoxic role. GDNF leads to a decrease in apoptosis through reducing inflammatory factors such as TNF-α and IL-1β and, finally, causes cell survival of neurons and improves memory and learning (3, 34).
It has been shown that the content of GDNF in the sciatic nerve (35), substantia nigra (36), and striatum (37) increases significantly following 12 and 18 weeks of moderate-intensity exercise. In contrast, two other studies reported that four weeks of low-to-moderate exercise training did not affect the protein content of the striatum and substantia nigra (38) and the GDNF content of the lumbar spine of rats (39). Therefore, increasing the expression of GDNF and improving learning can be influenced by the duration of exercise (3). Previous evidence and our findings show that regular exercise with moderate intensity causes some kind of adaptation in the antioxidant system, can increase resistance to oxidative stress, and prevent excessive excitability of neurons. Also, as a strong factor, it can play a role in cell proliferation of neurotrophic factors and suppression of accelerating factors in the process of neuronal destruction diseases, including inflammation caused by the activity of microglia and inhibition of NMDA receptors (2).
5.2. TRPV1 Receptor Distribution with Exercise Training in Epilepsy
Our results showed that the increase in TRPV1 expression in the EX, EX+PTZ, and EX-PTZ groups was significantly different compared to the PTZ group. It has been found that TRPV1 receptor protein expression increases as a result of moderate-intensity exercise. Sensory nerves adapt to exercise by increasing the expression of the TRPV1 receptor protein (40). There is increasing evidence that exercise increases NGF and neurogenesis (41). Recent reports have shown that NGF is upstream of the TRPV1 channel and increased NGF levels facilitate TRPV1 expression (42). On the other hand, there are also reports that NGF decreases in the DRG of rats with heart failure due to a decrease in muscle volume (43). At the same time, along with the decrease in NGF level, a decrease in TRPV1 levels has also been observed, and the evidence indicates a relationship between these two neural factors (44).
The relationship between delayed muscle pain and TRPV1 and the essential role of this receptor in delayed pain have been reported that usually occurs hours after intense or long-term activities. The results showed that delayed muscle soreness after the prolonged contraction was not increased in mice without TRPV1; however, the level of mRNA related to NGF increased three hours after muscle activity. These findings indicate that NGF upregulation is not impaired in the absence of TRPV1. Also, TRPV1 expression is NGF downstream in delayed muscle pain conditions (45). Due to the prominent role of this receptor on pain, the TRPV1 channel is one of the therapeutic targets for pain relief methods, which is done either by desensitization of the channel with an agonist or by blocking the channel with the use of an antagonist (46). Dysfunction of the synaptic plasticity of TRPV1 receptors has been reported in diseases such as Alzheimer’s, Parkinson’s, and epilepsy (47). Since the role of sports activities in increasing the expression of NGF has been confirmed in several reports, it can be concluded that the increase in the expression of NGF in cell signaling pathways and calcium-dependent pathways is related to the increase in the expression of the TRPV1 receptor, which is a cation dependent receptor. From a functional point of view and according to the available evidence on the relationship between TRPV1 and some sports biomarkers, it can be concluded that the role of this receptor is significant in regulating the factors involved in energy production. The findings of this research show that it is possible to consider TRPV1 as a neural marker in adaptation to exercise.
5.3. Signaling Correlation Between NGF and TRPV1
The results of our study showed a high correlation between NGF and TRPV1 in the CA1 region. Research results have shown that among the neurotrophic factors, NGF plays a more fundamental role in regulating the expression of TRPV1 and is involved in the expression of TRPV1 in the posterior root ganglion (DRG) (48). Exogenous NGF increases TRPV1 mRNA content, transport, and presence in the plasma membrane, as well as sensitivity to capsaicin in DRG neurons in a culture medium. Also, NGF upregulates TRPV1 during inflammation (49). On the other hand, in cases where nerve cutting occurs, such as axotomy of the sciatic nerve, downregulation of TRPV1 is seen, which is probably due to the lack of NGF. It determines the importance of the presence of NGF on the expression of TRPV1 (50). The mechanisms through which NGF facilitates TRPV1 expression remained unclear. Recently, it has been shown that NGF activates a small protein, GTPase Ras, whose activation is essential for the regulation of TRPV1 expression. Ras activation is an important component of the downstream pathway of inflammatory TRPV1 induction by NGF. Also, NGF may regulate TRPV1 expression by modulating the membrane potential, which leads to a depolarizing change in the membrane potential (50). In addition, the role of TRPV1 receptors in epileptogenesis has been reported. Hippocampal TRPV1 receptors are highly expressed in people with epilepsy suffering from temporal lobe epilepsy (51). Functional disorders of TRPV1 receptors cause changes in seizure induction in an animal model of tonic-clonic epilepsy (52). In contrast, TRPV1 agonist delays epileptogenesis in the kainic acid model of epilepsy (53). Therefore, our findings suggest that exercise modulates TRPV1 function in sensory neurons by increasing NGF expression.
5.4. Conclusions
These findings suggest that increased level of neurotrophins following moderate-intensity exercise may be involved in the antiepileptic effect of exercise through modulation of TRPV1 pathway. This study’s findings may be another emphasis on regulating moderate exercise as a supplementary treatment for patients who suffer from epileptic seizures. Also, considering the role of physical exercise on TRPV1 modulation can point out new approaches that may lead to the development of a new class of antiepileptic drugs for clinical applications.
5.5. Limitations
The limitation of this study is that the kind of cells (glia or neurons) involved in the TRPV1 modulation have not been distinguished. It seems that more studies in this field will help understand the mechanisms underlying the beneficial effects of exercise.