1. Background
Repairing massive bone and cartilage loss remains a significant challenge for orthopedic surgeons. The regeneration of both cartilage and bone has proven to be difficult due to their distinct physiological structures and functions (1). Most defects are typically caused by factors such as physiological bone resorption secondary to tooth loss, trauma, bone pathologies, or infections, leading to osteochondral (OC) defects (2). Osteochondral defects affect the superficial cartilage region, intermediate calcified cartilage, and subchondral bone, severely impacting patients' health and quality of life (3). One of the key objectives of Bone Tissue Engineering (BTE) is to design biodegradable scaffolds with appropriate porosity that integrate natural elements and molecular cues to foster tissue regeneration (4). Umbilical stem cells (USCs) have shown potential in promoting regeneration. Recent advancements in strategies for isolating, expanding, and shortening the timing of USC engraftment have significantly enhanced transplantation efficacy (5).
Bone formation consists of a series of complex events during the differentiation of mesenchymal stem cells into osteoblasts. Bone morphogenetic proteins (BMPs) play a crucial role in osteoblast differentiation by promoting the production of bone-specific matrix proteins (6). BMP-2, a key growth factor in the BMP subfamily, regulates osteoblast differentiation by stimulating osteoblast-related transcription factors, including runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), Collagen I (Col 1) (7), Collagen 10 (Col X) (8), and SOX9 (9, 10).
In the last two decades, several therapeutic approaches have been explored for bone regeneration, including scaffold-free and cell-based methods, as well as advanced treatments combining cells with various biomaterials (11). Biological scaffolds, derived from decellularized tissues and organs, have garnered significant attention because they can provide mechanical and chemical signals that promote cell attachment and differentiation in tissue regeneration (12). These scaffolds are influenced by both physical and procedural decellularization factors (13). Non-synthetic polymers, such as collagen, chitosan, starch, hyaluronic acid, and alginate, tend to have weaker and softer structures compared to ceramics but are recommended due to their flexibility and ability to conform to various shapes (14). Moreover, naturally sourced polymers can protect and guide cells during multiple stages of their proliferation, thanks to the presence of specific molecular domains, leading to biological interaction between the scaffold and the host tissue (7, 15).
Platelet-rich fibrin (PRF) is considered a platelet product and fibrin network that contains growth factors, cytokines, and cells. These factors are gradually released over time, making PRF useful as a biological membrane in various surgical applications (8). Osteochondral tissue engineering has shown significant progress in developing techniques for regenerating damaged cartilage and bone tissues.
Due to the limited self-repair capacity of cartilage tissue, it is essential to develop biomaterial-based approaches with different structures. Given the role of key genes in osteoregeneration and the growing need for more cost-effective, rapid, and novel bone regeneration methods, this study evaluated the histological impact of acellular scaffolds in combination with stem cells derived from cord blood and platelet-rich fibrin.
2. Objectives
Additionally, the expression of Col 1, Col X, Runx2, SOX9, and ALP genes was investigated in the repair of knee defects in rabbits.
3. Methods
3.1. Prepration of Acellular Scaffolds
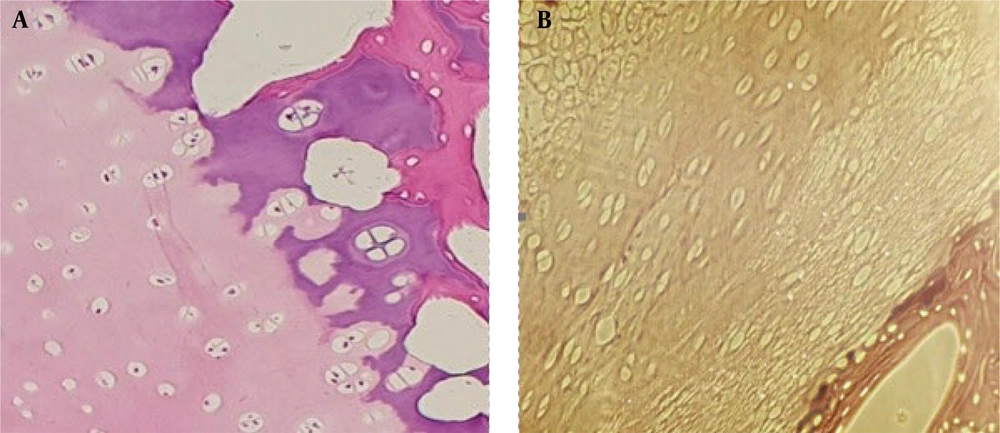
Osteochondral samples were obtained from the femoral patella of ovine bone marrow and transferred to the laboratory for freeze-thaw processing. Initially, the fragments were washed twice with phosphate-buffered saline. The samples then underwent four freeze-thaw cycles, where they were frozen at -121 degrees Celsius for 20 minutes and subsequently placed in liquid nitrogen. After each cycle, the blocks were washed with distilled water. To remove cellular debris, the blocks were treated with 1% Triton X-100 for 8 hours, followed by an additional treatment for 16 hours. To eliminate any remaining detergent, the samples were washed twice with distilled water for 24 rounds. The entire procedure was carried out using a rotator at room temperature.
3.2. Platelet-Rich Fibrin Preparation
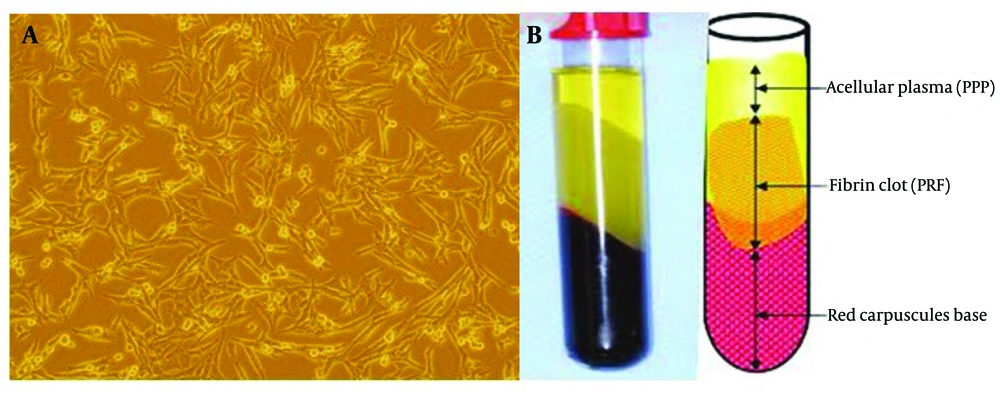
Platelet-rich fibrin was prepared by drawing 20 mL of blood from a single candidate and transferring it into 10 mL sterile tubes without any anticoagulant. All samples were centrifuged at 2700 rpm for 3 minutes. The upper layer of each tube was collected and stored in sterile syringes for scaffold fabrication. Subsequently, the PRF structure was lyophilized and stored in a -20°C freezer until use.
3.3. Cell Culture
After receiving USCs from healthy donors, they were transferred to the laboratory for stem cell isolation processing. Initially, the umbilical cord was washed three times with phosphate buffer saline, then fragmented and digested with type I collagenase for 30 minutes at 37°C. The cells were treated with DMEM-low glucose (1 g/liter) and incubated at 37°C. After 24 hours, the adherent cells were cultured and prepared for the study.
3.4. Loading the Different Combination of Stem Cells Derived Cord Blood and Platelet Rich Fibrin on Acellular Scaffolds
The scaffolds were wetted in DMEM medium and incubated for 1 hour. After removing the medium, 1 × 106 stem cells derived from cord blood and platelet-rich fibrin were loaded onto the scaffolds and incubated for an additional 2 hours.
3.5. Preparation of Animal and Histology
In this study, 12 male New Zealand rabbits, with a mean weight of 1.300 - 1.500 kg, were housed in the animal facility at Tehran Medical University, following approval from the Ethics Committee (Approval ID: IR.TUMS.AEC.1402.070). A hole measuring 4.5 mm in diameter and 4 mm in depth was drilled in the femoral patella of each rabbit's knee. The rabbits were divided into four groups (3 rabbits per group): (1) control (OC), (2) acellular osteochondral (AO), (3) acellular osteochondral + PRF, and (4) acellular osteochondral + stem cells derived from cord blood/platelet rich fibrin. The defect in the femoral patella was treated with scaffolds in groups 2, 3, and 4. Six months post-operation, the implanted tissues were harvested and transferred to 10% neutral buffered formalin (NBF, pH 7.26) for 48 hours. The samples were then decalcified in 10% formic acid for 10 days and processed. Sections of 5µm thickness were stained with Hematoxylin and Eosin (H&E), Masson’s Trichrome (MT), and Safranin O (SO).
3.6. Analysis of Real time Polymerase Chain Reaction (PCR)
Using Trizol (Invitrogen, USA), total RNA was extracted. The RNA purity was assessed by measuring the absorbance at 260 nm and 280 nm (Nanodrop, Biotek, USA). The extracted RNA was then reverse transcribed into complementary DNA (cDNA) using a Takara kit (Japan). The expression levels of mRNA transcripts were quantified through real-time PCR (Rotor Gene 6000, Corbett, Australia). The PCR product detection was based on the fluorescence enhancement observed from the binding of SYBR Green to double-stranded DNA. Specific primers were used for the following genes: SOX9 (forward: 5’-GGCAGCTGTGAACTGGCCA-3’, reverse: 5’-GCACACGGGGAACTTGTCC-3’) (18), collagen type I (forward: 5’–CGGCTCCTGCTCCTCTTAGCG–3’, reverse: 5’–CTGTACGCAGGTGACTGGTG–3’) (18), Runx2 (forward: 5’–GACTGTGGTTACCGTCATGGC–3’, reverse: 5’–ACTTGGTTTTTCATAACAGCGGA–3’), Col X (forward: 5’–GAAAACCAGGCTATGGAACC–3’, reverse: 5’–GCTCCTGTAAGTCCCTGTTGTC–3’), and ALP (forward: 5’–TGTGCGGGGTCAAGGCTAAC–3’, reverse: 5’–GGCGTCCGAGTACCAGTTGC–3’) (19), which were designed using Primer3 software. The following PCR conditions were applied: 94°C for 15 min, followed by 45 cycles at 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. GAPDH (forward: 5’–CTGGTGCTGAGTACGTGGTG–3’, reverse: 5’–CGTCAGCAGAAGGTGCAGAG–3’) was used as a housekeeping gene to normalize the RNA quantity and quality.
3.7. Statistical Analysis
The analysis of variance (ANOVA) was utilized to assess the significance of new bone and blood vessel formation, using GraphPad Prism software, Version 6.00 (GraphPad Prism, Inc., San Diego, CA). A significance level was set at P < 0.05.
4. Results
4.1. Acellular Scaffolds and Isolation of Stem Cells Derived Cord Blood and Platelet Rich Fibrin
The cells from the sample fragments were removed, and the scaffolds were prepared for implantation. The decellularized scaffold was confirmed histologically (Figure 1). Stem cells derived from cord blood and plasma rich fibrin were isolated from the blood, and the scaffolds were exposed to these components for implantation (Figure 2).
4.2. Histopathological and Radiological Analysis
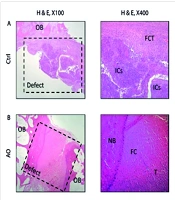
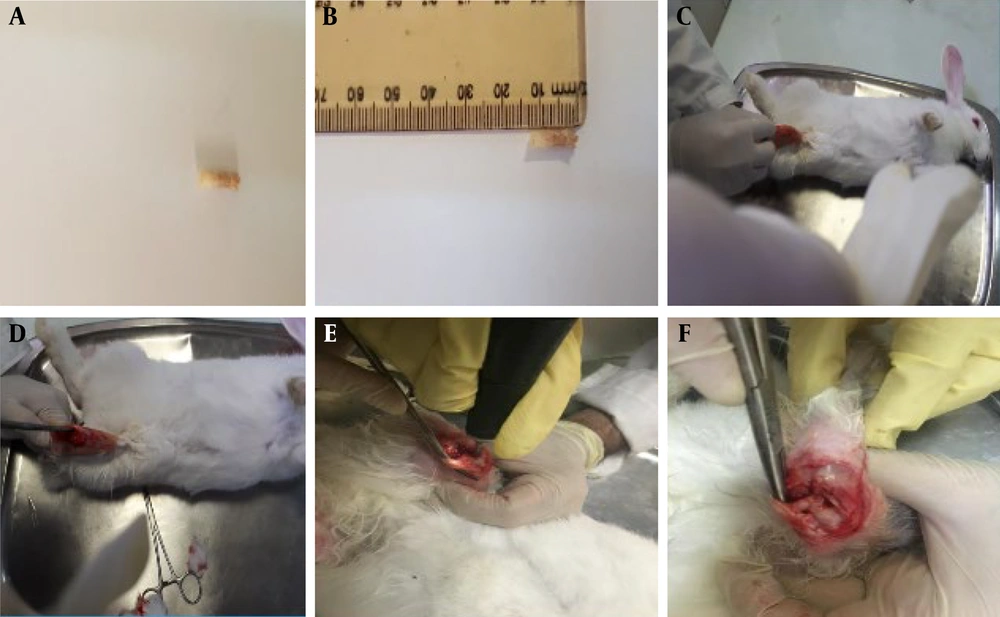
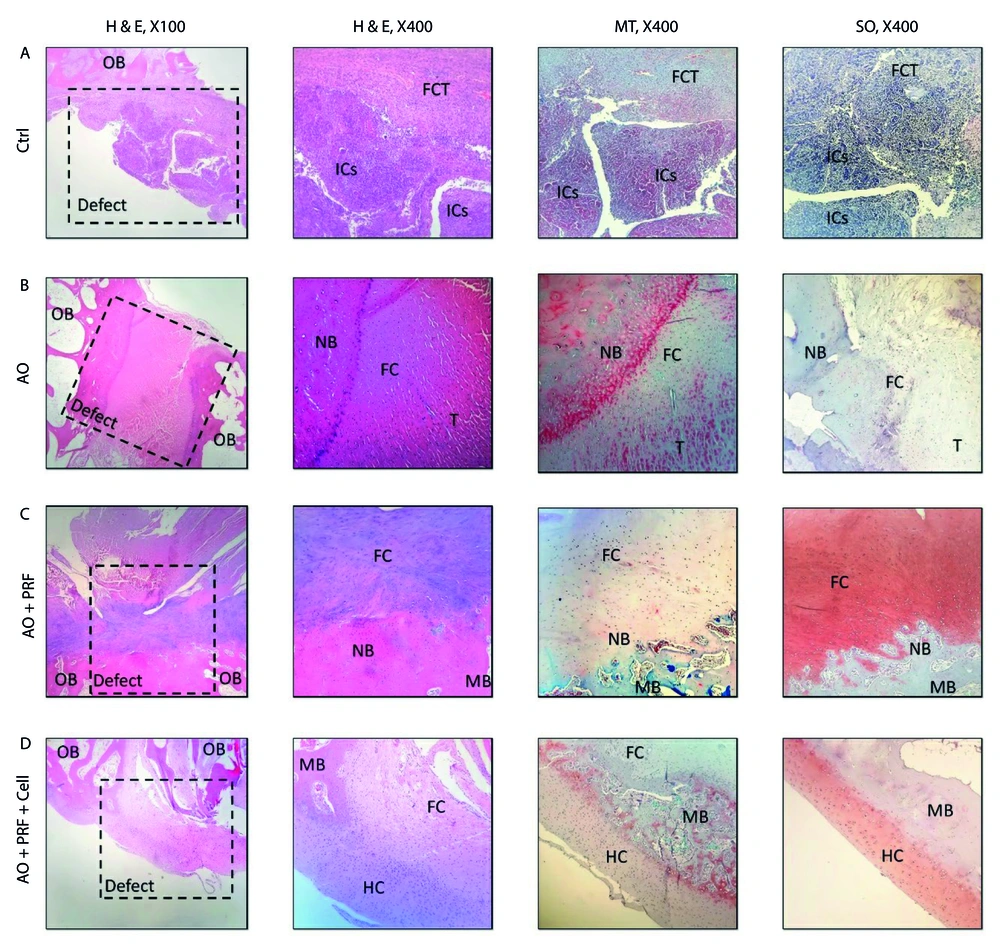
The scaffolds were implanted into the femoral patella of rabbits in groups 2, 3, and 4. As illustrated in Figure 3, the various stages of implantation include: A and B show the acellular scaffold, C and D depict the skin incision, E shows the muscle incision, and F illustrates the implantation of the scaffold. Histological sections were examined 6 months post-implantation. In the control group (untreated), the defect site was completely repaired with fibrous connective tissue (FCT), and an accumulation of inflammatory cells (ICs) was evident. In the group treated with the acellular osteochondral graft, the defect area was replaced with a combination of neo-bone and fibrocartilage. Additionally, tendon-like tissue (connective tissue) had accidentally entered the defect site. The immaturity of fibrocartilage tissue was confirmed using the Safranin O (SO) staining technique. The stained images of the osteochondral area in the AO+PRF-treated group showed the formation of new bone and cartilage, with a significantly higher amount of cartilaginous tissue compared to normal tissue. Furthermore, mineralization of the fibrocartilage tissue was observed on a large scale. In the AO + PRF + cell group, new bone maturation was considerably higher than in other groups. The implanted material was fully recellularized, and most of the desired tissue was hyaline cartilage, with the cartilage layer showing normal thickness (Figure 4).
The findings of Histopathological in defect of osteochondral in the experimental groups. A, in the control group (untreated), the area of osteochondral defect was completely replaced with fibrous connective tissue (FCT) and the accumulation of inflammatory cells (ICs) was evident; B, in Acellular Osteochondral graft, the implanted graft completely filled by two types of tissue: Neo-bone and immature fibrocartilage (SO staining); C, the osteochondral defect in OA + PRF treated group showed new bone and cartilage formation, however, the amount of cartilaginous tissue was significantly higher than normal tissue; D, the maturation of new bone in OA + PRF + cell treated animals was significantly higher than other treatment groups. In addition, the thickness of the cartilage layer was normal. OB: Old Bone, NB: New Bone formation, ICs: Inflammatory Cells. FC: Fibrocartilage, HC: Hyaline cartilage, FCT: Fibrous Connective Tissue, T: Tendon-like tissue, MB: Mature bone.
Overall, no foreign body reactions or severe inflammatory responses were observed. The AO+PRF-cell and AO+PRF groups exhibited significant new bone ingrowth (65 ± 6%), whereas the free AO group did not show significant new bone formation (4 ± 1%). Hyaline cartilage was formed in the AO+PRF-cell treated group. The amount of new tissue was significantly higher in the AO+PRF (158 ± 13) and AO + PRF + cell (1 ± 11) groups compared to the AO (62 ± 8) and control groups, as determined by histomorphometric analysis (Table 1). This demonstrated good biocompatibility in all experimental groups (Table 2).
| Groups | New Cartilage Tissue (%) | New Bone Formation (%) |
|---|---|---|
| Ctrl | 0 A | 1.6 ± 1.1 A |
| AO | 10.3 ± 2.5 B | 18 ± 2 B |
| AO + PRF | 43 ± 4.3 C | 45.3 ± 5.1 C |
| AO + PRF + Cell | 48 ± 5 C | 32 ± 3.6 C |
4.3. Chondrogenic Gene Expression Assessment by Real time PCR
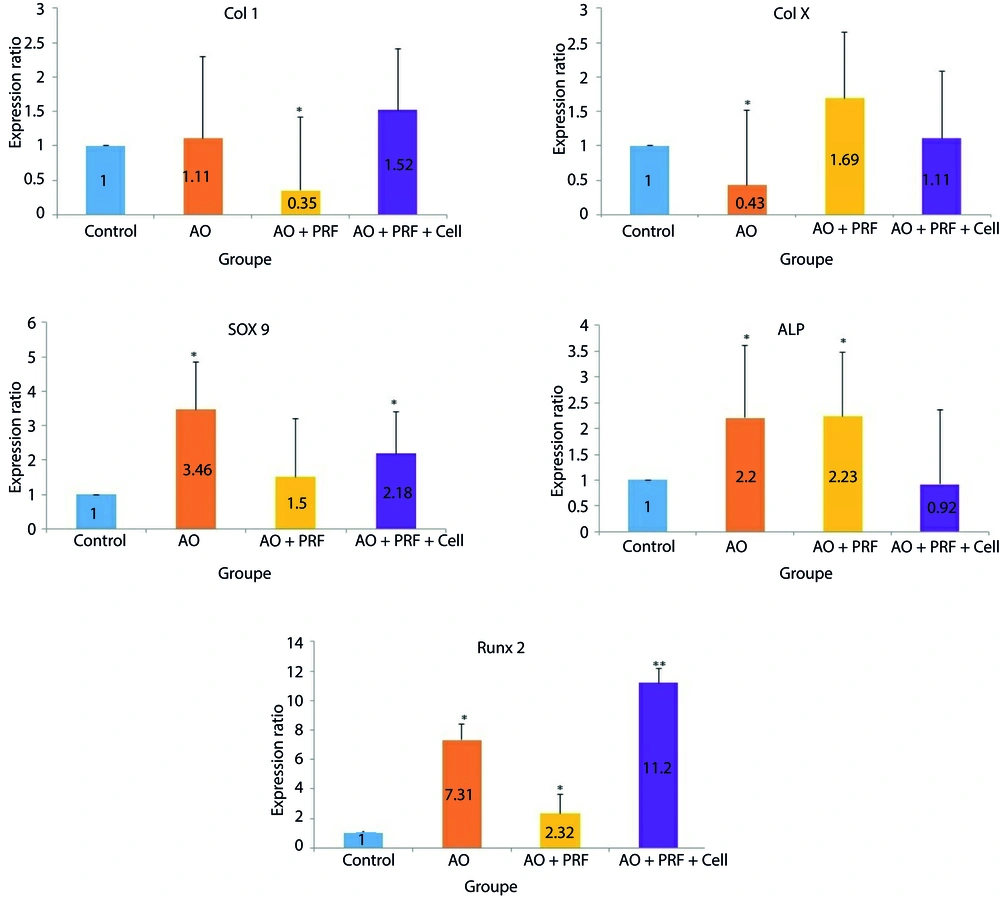
The results of Col 1 gene expression showed a significant decrease in the AO + PRF group (P = 0.003) compared to the Control group (0.25 or 2.86-fold). The other groups showed an increase: AO group by 1.11-fold and AO + PRF + Cell group by 1.52-fold; however, these increases were not statistically significant, with p = 0.649 and P = 0.137, respectively, compared to the Control group.
The expression level of Col X gene in the AO group demonstrated a significant decrease (P = 0.005) compared to the other three groups. Although the level of Col X increased in the AO + PRF group, the change was not statistically significant (P = 0.325). The effect of AO and AO + PRF + Cell on the mRNA expression of SOX9 was significant with P = 0.012 and p = 0.036, respectively.
The ALP gene expression showed a significant increase in both the AO group (P = 0.039) and the AO + PRF group (P = 0.024) compared to the Control group.
Additionally, the Runx2 gene expression showed significant changes across all groups compared to the Control group. The AO group had a 7.31-fold increase (P = 0.000), the AO + PRF group had a 2.32-fold increase (P = 0.031), and the AO + PRF + Cell group had an 11.2-fold increase (P = 0.000) (Figure 5).
The graphs show the expression of Col 1, Col X, SOX9, ALP and Runx 2 in 3 groups (AO (acellular osteochondral), AO + PRF (acellular osteochondral + platelet rich fibrin) and AO + PRF + cell (acellular osteochondral +stem cells derived cord blood/ platelet rich fibrin)) compared with the control group. Overall, the expression of Col 1 in AO + PRF group, Col X in AO group, SOX9 in AO and AO + PRF + Cell group, ALP in AO and AO + PRF group and Runx2 in all group have shown significant difference compared with Control group. Data are determined as means ± SD; *P < 0.05; **P < 0.01.
5. Discussion
Our results have shown that the simultaneous use of scaffolds, such as acellular osteochondral, with stem cells like stem cell-derived cord blood and PRF, can effectively accelerate the regeneration process for OC defects in animal models. As observed in the histological analysis, the maturation of new bone and cartilage in the OA + PRF + Cell group was significantly higher compared to the other groups. Additionally, our radiological results indicated that the healed bone exhibited less density when PRF was used as the sole filling material, especially when combined with stem cells. This outcome is expected, as PRF is considered a membrane with soft tissue density (16). In a similar study conducted by Zhang et al. in 2017, it was demonstrated that platelet concentrations in PRP samples were 4.9 times higher than in whole blood samples, and the histological analysis of neo-tissue in samples treated with scaffolds and autologous PRP showed subchondral bone formation in a rabbit model (17).
Recently, natural scaffolds have garnered increased attention due to their ability to provide mechanical and chemical signals for cell attachment and differentiation in tissue regeneration. Scaffolds such as platelet-rich plasma (PRP) and PRF are new biological tools in tissue engineering and are widely used by surgeons and dentists. Moreover, various studies have shown positive outcomes with the application of PRF, either as a sole filler or in different combinations, delivering satisfactory results (18, 19).
In this study, we evaluated the expression of five genes involved in articular cartilage degeneration, osteoarthritis, repair, regeneration, and transplantation. Col I is an osteogenesis-related gene and serves as an ideal marker for bone regeneration (20-22). In this study, the expression of the Col I gene showed a significant decrease in the AO + PRF group, indicating a reduction in tissue degeneration within this group.
SOX9 is a key cartilage matrix protein that regulates and expresses itself alongside type II collagen (Col II) and Col I (23). Researchers have demonstrated that collagen type I and X (COL I, COL X) genes express hypertrophy-related proteins (24). The Col X content was lower in the AO group, which correlated with enhanced bone generation (25).
In terms of ALP gene expression, the AO + PRF + Cell group showed a decrease compared to the AO and AO + PRF groups. ALP is considered an early marker of osteoblast differentiation and tends to increase in osteochondral defects (26). However, the simultaneous implantation of acellular osteochondral scaffolds, stem cells, and plasma-rich fibrin was shown to reduce this expression in the animal model.
On the other hand, Runx2 gene expression significantly increased in all three groups. During the development of osteochondral defects, the hypertrophic process occurs alongside the differentiation of articular chondrocytes. Runx2 plays a fundamental role in regulating key genes involved in chondrocyte/osteoblast differentiation and matrix degradation (27, 28). Many studies have demonstrated that Runx2 expression levels are elevated in human osteochondral defects. To investigate the role of Runx2 in the development of osteochondral defects, genetic animal models have been utilized. Overexpression of Runx2 in mice has been shown to increase the expression of cartilage proteases in chondrocyte cells (29, 30). This also leads to the activation of matrix degradation enzymes, such as MMP13 and ADAMTS5, through the mitogen-activated protein kinase (MAPK) pathways (31), and directly regulates MMP13 gene transcription (32). It is now understood that the implantation of scaffolds, along with a large number of cells, is necessary for the repair of osteochondral defects. However, Hansen et al. reported that increasing cell seeding density did not have a positive effect on cartilage repair (33). The results of this study indicate that selecting the best materials is crucial for the reconstruction process, which requires further research in the field of tissue engineering.
5.1. Conclusions
In conclusion, the use of stem cells and plasma-rich fibrin in combination with natural scaffolds, such as acellular osteochondral scaffolds, can serve as an effective filler for repairing bone and cartilage in medical applications. However, evaluating more genes and proteins in other animals and different parts of the skeletal system could provide a deeper understanding of these biomaterials.