1. Context
Traumatic spinal cord injury (SCI) results in damage to the spinal cord parenchyma and associated nerves, leading to loss of sensory, motor, and autonomic functions (1, 2). Spinal cord injury pathophysiology categorizes these injuries as primary (due to mechanical forces) and secondary injuries (resulting from ischemia, inflammation, and other pathways) (3).
The prevalence of SCI has risen over the past three decades, with males and elderly individuals being significantly more affected. The World Health Organization (WHO) notes that between 13 and 33 million people worldwide have subsequent SCI, and there are 250,000 to 500,000 new cases annually. In the United States, the incidence is estimated at 54 cases per million people each year, contributing to healthcare costs exceeding $1.69 billion annually (1). The global impact of SCI can be reduced through effective prevention, treatment, rehabilitation, and continuous healthcare (4). Current therapeutic options for SCI are limited, leaving patients with lifelong physical and mental health issues (1, 3, 5). However, developing effective therapies for SCI recovery requires improved comprehension of the initial and secondary mechanisms of injury, as well as regenerative pathways.
Several animal models have been developed to evaluate the anatomical and biological aspects of SCI to improve care (5). Animal models are essential for in vivo SCI research, as they provide controlled environments for studying physiological and pathological processes. To develop effective treatments, these models need to accurately mimic human conditions (6). Vertebrate animals can regenerate their spinal cords during early development, but their regenerative abilities in adulthood vary among phylogenetic groups. Although rodents are the most common species used in SCI models, other vertebrates such as fish have also been studied due to their distinctive regenerative capacities (5).
This review was performed to better understand the use of fish species as models for SCI research. Specifically, this manuscript categorizes the diverse methodologies and outcomes associated with using fish models, including their regenerative capacity.
2. Objectives
This systematic review classifies fish models for SCI based on study purpose, injury patterns and grades, outcome metrics, and fish species.
3. Evidence Acquisition
3.1. Electronic Searches
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. A comprehensive literature search was conducted using PubMed, Scopus, Web of Science, and Embase databases. Medical Subject Headings (MeSH) terms and keywords such as "fishes," "goldfish," "spinal cord injuries," "spinal cord," "spinal injuries," "wounds and injuries," and "zebrafish" were employed to identify relevant articles up to July 2023. A detailed search strategy is included in Appendix 1 in Supplementary File. Google Scholar and reference lists of primary studies and related reviews were manually screened to capture additional relevant articles. The Ethics Committee of Sina Trauma and Surgery Research Center at Tehran University of Medical Sciences approved the study protocol (reference number: IR.TUMS.SINAHOSPITAL.REC.1400.073).
3.2. Eligibility Criteria
This review included all observational and interventional studies published in peer-reviewed journals that used fish species as a model for SCI. Studies that focused on amphibians or used fish models for peripheral nerve injury were excluded. Reviews, abstracts, and editorial articles were also excluded from the analysis.
3.3. Selection and Data Collection Process
Two independent reviewers conducted the initial title-abstract screening and subsequent full-text evaluation of potentially eligible studies. Relevant studies were recorded in a predefined data collection sheet (Appendix 2 in Supplementary File). Any discrepancies between the reviewers were resolved through adjudication with the corresponding author.
3.4. Assessment of Quality and Risk of Bias (RoB)
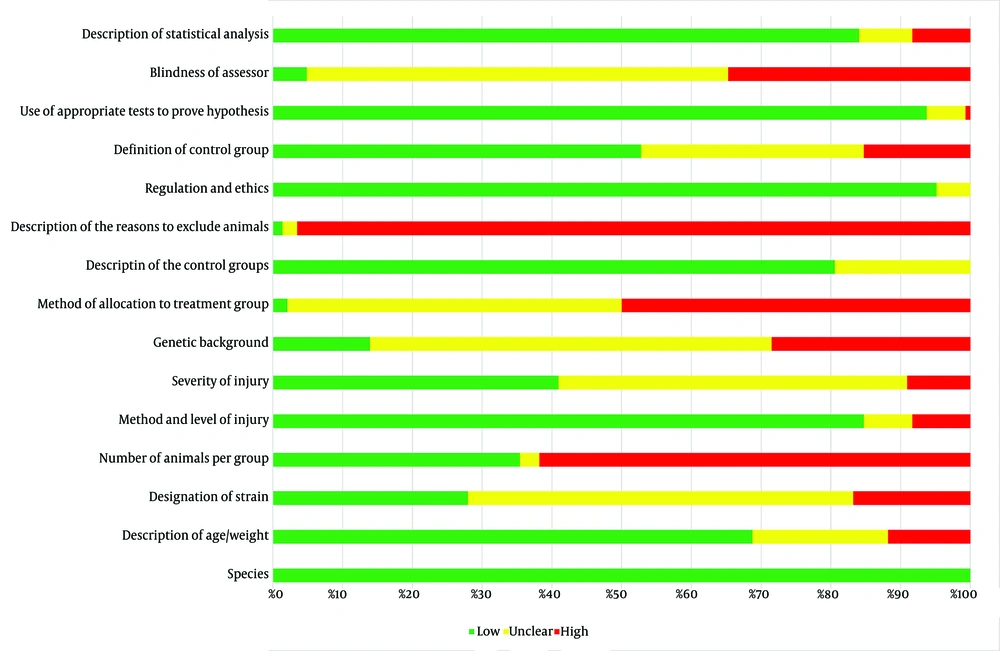
The risk of bias in the included studies was assessed by two independent reviewers using the RoB assessment tools designed for animal studies in SCI research (7). Studies that met at least eight of the 15 internal validity criteria were considered high-quality or low-bias. Out of the 144 animal studies reviewed, 101 were classified as high-quality, and 43 were identified as low-quality (Figure 1).
The risk of bias assessment of the included studies based on the Hassannejad et al., 2016 study (7). Each row contains a variable that checks through the included studies and scores them based on high, unclear, or low risk of bias.
4. Results
4.1. Description of Studies
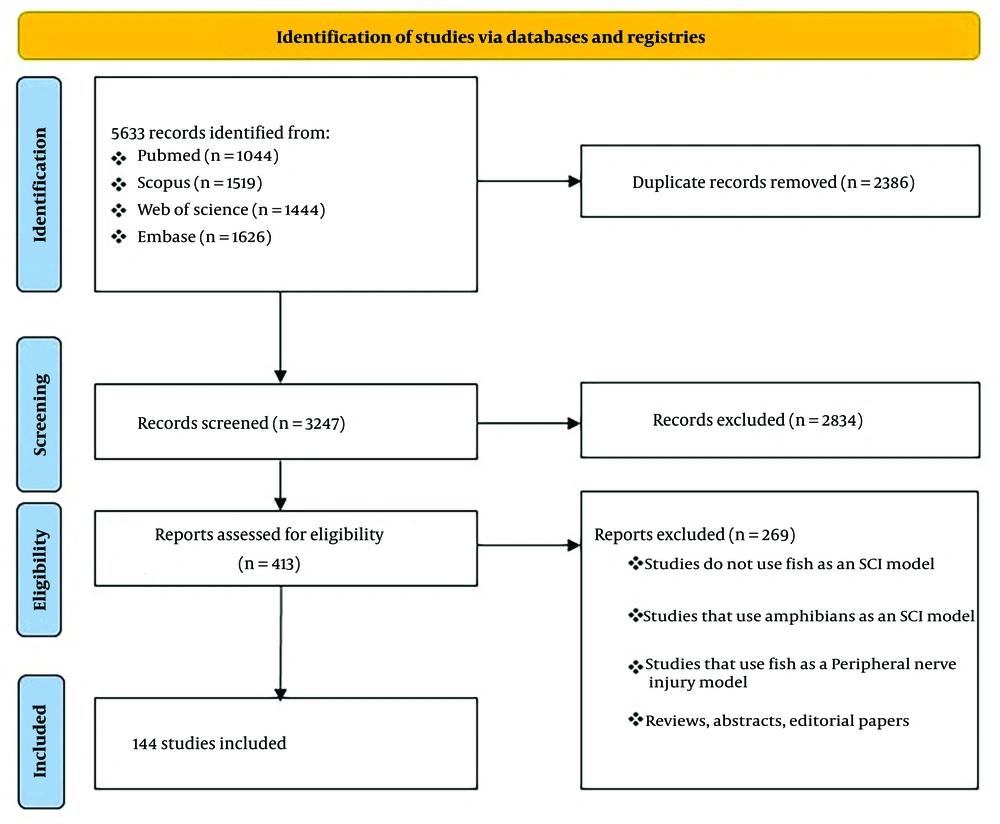
A total of 5,633 records were identified through title and abstract screening, of which 413 manuscripts underwent full-text review. After full-text analysis, 144 studies were included in the final analysis (Figure 2).
These studies were categorized based on the fish species used as SCI models:
Zebrafish (Danio rerio): One hundred and two studies
Lamprey (Petromyzon marinus and Lethenteron reissneri): Thirteen studies
Goldfish (Carassius auratus): Eleven studies
European eel (Anguilla Anguilla): Nine studies
Knifefish (Apteronotus leptorhynchus and Apteronotus albifrons): Six studies
African turquoise killifish (Nothobranchius furzeri): Two studies
Sailfin Molly (Poecilia latipinna): One study
In the 1920s, studies on goldfish (Carassius auratus) and crucian carp (Carassius carassius) showed functional recovery after SCI, although no notable morphological investigations supported these findings at the time (8). Subsequent research on species like guppy (Poecilia reticulata) and Japanese rice fish (Oryzias latipes) demonstrated that nerve fibers reconnect the severed spinal cord, leading to the restoration of swimming behavior (9). However, systematic exploration of fish models for SCI remains limited.
Spinal cord transection was the most common method used to induce spinal cord trauma in fish models. Tail amputation was another prevalent method for inducing injury, particularly in fish species that can regenerate entire tail structures, which is phylogenetically distinct from mammals that do not have tail segments. Thus, spinal cord transections are considered more consistent with mammalian SCI models.
Another injury method involved laser axotomy, which was used in both larval and adult zebrafish across seven studies (10-16). Additionally, electroablation was employed in one study to induce spinal cord neural injury (17). In this technique, microelectrodes were positioned between the horizontal myoseptum and dorsal ridge of zebrafish larvae, and a 25 μA pulse was applied for 1 second to inflict damage (18).
One study used a substrate-induced method to damage the spinal cord by injecting α-bungarotoxin into the junction between the brainstem and spinal cord of adult zebrafish (17). For chemical demyelination, Cunha et al. (19) utilized lysolecithin. The results from studies employing these various methods to induce SCI, including those investigating axonal regeneration at the injury site, are consistent with findings from more commonly used techniques.
4.2. Zebrafish
Of the 102 studies that utilized zebrafish as an SCI model, 30 involved zebrafish larvae. The primary mechanism of injury in these studies was spinal cord transection. Fifteen studies employed alternative injury mechanisms, such as laser-induced spinal avulsion (10-16), electroablation using tungsten microelectrodes (18, 20-22) and pulled glass microelectrodes (23), and demyelination using substrates such as lysolecithin (19, 24) and α-bungarotoxin (17). The most common injury site was located 3 - 5 mm caudal to the brainstem-spinal cord junction, typically at the level of the 15th myotome. Other injury locations included the anal pore (22, 25, 26), optic tectum (27), 7th segment (28, 29), 16th-18th somites (24), and 17th - 20th somites (30). All these studies consistently described the injury as complete axotomies (Appendix 3 in Supplementary File).
This analysis revealed that zebrafish are the predominant fish species utilized for traumatic SCI models. Zebrafish are a well-established model for vertebrate development due to their embryonic transparency, which allows for easy observation of developmental processes. Furthermore, genomic sequencing has enabled the development of molecular assessment tools for detecting neuronal regrowth (31).
Following spinal cord transection in zebrafish, approximately 8% of actively dividing cells differentiate into motor neurons near the injury site (32). In adult zebrafish, the spinal cord undergoes significant structural and functional changes after injury, leading to the eventual restoration of normal function. These acute and chronic regenerative responses result in functional recovery around six weeks post-lesion (WPL) (33), with no additional improvement observed after ten WPLs (34). Upper motor neurons located in the nucleus of the medial longitudinal fascicle and intermediate reticular formation have the ability to repair their axons within the spinal cord. This regeneration is facilitated by the early activation of anti-apoptotic molecules. Regenerating axons from higher motor neurons migrate to the injury site within 10 - 15 days, crossing it within 4 - 6 weeks post-SCI. This process leads to an increase in anti-apoptotic molecules, including Bcl-2 and phospho-Akt, within 1 - 6 days after SCI (35).
Retrograde tracing studies have shown significant changes in dopaminergic and serotonergic systems during successful spinal cord regeneration in adult vertebrates. One study focused on localizing dopamine signals and revealed that Th1+ axons originating from the brain are the primary source of dopamine in the spinal cord (35). After a spinal cord lesion, significant dopaminergic system alterations occur, initially reducing rostral to the lesion and then continuously increasing with regrowth caudal to the lesion. The majority of Th1+ axons are dopaminergic, and their regrowth correlates with functional recovery. In the serotonergic system, circuitous axons with active synapses were observed, and lesion-induced changes included a reduction rostral to the lesion and a subsequent increase caudal to the lesion. The number of 5-HT+ axons caudal to the lesion correlated with the recovery of distal function. These findings highlight the plasticity and regenerative potential of the dopaminergic and serotonergic systems after SCI in zebrafish (35).
4.3. Lamprey
Thirteen studies employed Sea Lamprey (Petromyzon marinus) and Asiatic Brook Lamprey (Lethenteron reissneri) as their SCI models, with ten using Lamprey larvae. The predominant injury mechanism was spinal cord transection at the 5th gill level. The severity of the injury in these studies was consistently described as complete, with no partially injured models employed (Appendix 4 in Supplementary File).
Using gamma-aminobutyric acid (GABA) immunofluorescence, Romaus-Sanjurjo et al. (36) monitored anatomic changes at the transection site of mature lamprey larvae for 10 WPL. There was a notable reduction in GABA cells and fibers observed one hour after the injury at both the rostral and caudal directions to the lesion. The numbers of GABAergic cells and their innervation returned to control values within 1 to 2 WPL. The expression of transcripts for GABA type B receptor subunits 1 and 2 was considerably reduced in the spinal cord with lesions compared to control animals at 1, 4, and 10 WPL.
4.4. Goldfish
Eleven studies employed goldfish as their SCI fish model, with five studies using adult fish. The primary injury mechanism was spinal cord transection, although some studies employed variations such as crushing injury (37) and hemisection (38). The injury sites varied, including the left side of the spinal cord (39), spinomedullary level (40, 41), first spinal nerve (38), and posterior median septum (42). Consistent with the other fish species, the fish received complete injuries, except for two studies with the crush and hemisection injuries (Appendix 5 in Supplementary File).
In mammals, after SCI, the extracellular matrix inhibits neuroregeneration (43). Interestingly, these same proteins are present at the injury site in goldfish up to six WPL but do not interfere with neurogenesis (44). One such protein is chondroitin sulfate proteoglycans, which are present at the lesion site but do not obstruct the growth of regenerating axons in goldfish, as opposed to mammals. Neurites from the goldfish midbrain nucleus grow toward the spinal cord after the injury, promoting neurogenesis by innervating the spinal locomotor neurons. This re-establishment of locomotor nucleus neurons with their correct distal pathways may serve as the foundation for enabling functional recovery in goldfish (39). Two studies revealed the aberrant pathway choice of the Mauthner axon in the recovery of behavior and reactive cell invasion after Mauthner axon injury (45, 46).
4.5. European Eel
Eels are considered fish and specifically categorized as ray-finned fish. Nine studies utilized the European eel as an SCI model, with two employing immature eels. The injury mechanism in all these studies involved the transection of the spinal cord. The most common site of spinal cord lesion was 13 segments caudal to the anus, with the exception that some studies injured four segments rostral to the anus (47), the level of the third vertebra (48), and the level of body segment 60 (49). All included studies consistently described the injury as complete, without any partially injured models (Appendix 6 in Supplementary File).
Flight and Verheijen (48) analyzed a European eel’s four distinctive behavioral rehabilitation stages—head and body movement, swimming behavior, rheotaxis, and shelter-seeking—to monitor neurogenesis development. These authors noted no differences from normal behavior in lesioned eels after six weeks, where behavioral recovery was considered complete. These findings align with Doyle et al. (50), who reported that fish with spinal injuries were able to regain their normal tail functions after 5 weeks.
4.6. Other Teleost Fish
Five studies used brown ghost knifefish (Apteronotus leptorhynchus) as their SCI model, all employing a transection mechanism of injury. In two studies, the site of the lesion was 4 mm caudal to the brainstem-spinal cord junction. One adult and one larval African turquoise killifish were used in two studies. The site of injury in the adult killifish was not related to SCI but was a contusion injury on the left optic nerve, whereas the larval killifish underwent a complete transection at the opposite site of the anal pore. Black ghost knifefish (Apteronotus albifrons) and sailfin molly fish (Poecilia latipinna) were used in one study each as complete transection SCI models (Appendix 7 in Supplementary File).
Sîrbulescu and Zupanc (51) monitored the adult brown ghost knifefish at two hours post-injection in the injury site. The caustic agent was active-caspase-3/bromodeoxyuridine/Hu triple labeling, serving as a quantitative analysis of apoptosis. Only 8% of cells showed signs of apoptosis, increasing to 16% during 1 - 3 days post-injury. Between days 5 - 100, only 2% of cells underwent apoptosis. After 150 days, the number of cells labeled with these three markers was only 10% of the total number of apoptotic cells, and by 200 days, no labeled cells could be identified. The increased apoptosis along the lesion margins may suggest that apoptosis plays a role in clearing non-functioning cells and promoting tissue healing during spinal cord regeneration, in contrast to traditional mammalian models where SCI-related apoptosis is viewed negatively as a process that eliminates cells post-injury.
In contrast to mammals, Vitalo et al. (52) reported that the glial scar in the SCI model of brown ghost knifefish forms a well-developed network of radial glia in both intact and wounded spinal cords. This network supports the regeneration of tissue lost to injury and likely plays a crucial role in generating new neurons.
5. Discussion
This systematic review highlights the diverse applications of fish models in SCI research and their potential to elucidate SCI pathophysiology and regenerative processes. The widespread use of spinal cord transection across various fish species emphasizes the value of these models for investigating axonal regeneration and recovery mechanisms. However, it is recognized that this injury model differs from the blunt trauma mechanism typically seen in human SCI. Zebrafish, with their transparency and advanced molecular tools, are particularly valuable for studying regenerative responses in the spinal cord, while goldfish and European eels demonstrate significant potential for overcoming inhibitors of neuroregeneration. The findings related to apoptosis and the formation of glial networks in teleost fish models offer valuable insights into tissue repair mechanisms.
Future research should focus on standardizing injury paradigms and protocols across different fish species to enhance the comparability and reproducibility of results in SCI studies. Moreover, investigating the molecular and cellular mechanisms underlying the regenerative capacities observed in fish models could offer promising avenues for developing targeted therapeutic strategies for treating human SCIs.
5.1. Limitations
There are significant variations in the methods and types of SCIs across different fish species and studies, making it challenging to compare findings and draw generalized conclusions. The diversity in study designs, species, and injury paradigms may also limit the generalizability of findings to the human SCI context. This review highlights the need for standardized protocols and injury mechanisms to improve comparability and reproducibility in future research involving fish SCI models.