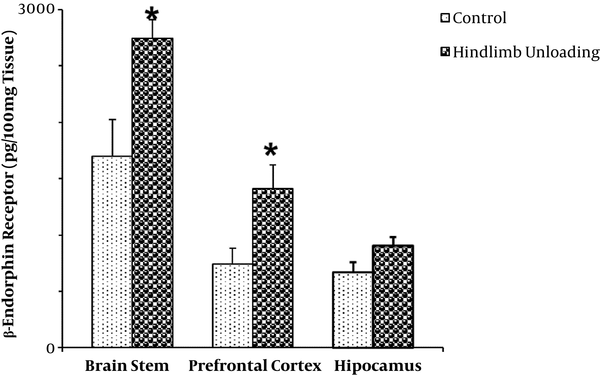1. Background
Performing the simulated experiments of weightlessness helps researchers to perceive unknown dangers and risks, which take place in an aircraft platform. These researches can be the way to create a shortcut to enter an important environment. Exposure to space flights is associated with several stress factors such as microgravity and circadian alteration. These conditions can affect the physiological function of human and animal organism (1, 2). These include cardiovascular, muscle and immune related problems, decreased bone mineral density, and circadian rhythm-related problems involving sleep and performance (3). In addition, space travel has an array of effects, which are the causes of changes in some activities namely eye movements, reflex control, respiratory, gastrointestinal, and CNS functions. The brain is affected by space flight, which has pivotal effects on that (4). Testing and research related to the conditions of weightlessness is the way to develop and expansion of space medicine. Space and weightlessness conditions, due to stress, can cause β-endorphins secretion from the brain and hypothalamus. The opioid system plays a central role in nociception and analgesia. The analgesic effect of β-endorphin is greater than morphine (5). It can also regulate numerous physiological functions, including responses to stress, respiration, gastrointestinal passage, and functions of endocrine and immune systems (6). Relaxation and mental health is one of the important factors during space flight, therefore having enough information to predict changes in behavioral and physiological events are necessary.
Opioid peptides and their receptors are widely expressed all over peripheral and central nervous system. β-endorphin receptors are broadly distributed throughout the human CNS, with a particularly dense distribution in the basal ganglia, cortical structures, thalamic nuclei, spinal cord, and specific nuclei in the brain stem (7-11). High levels of this receptor are found in the basolateral amygdala, nucleus accumbens, hypothalamus, thalamus, ventral tegmental area, and caudate putamen. β-endorphin receptors, in the periaqueductal gray matter mediate the anti-nociceptive effects of opioids. Many studies show that a variety of stressors activate β-endorphins in rodents (12).
2. Objectives
Current information is limited regarding changes of the content of neuropeptides and neurotransmitters in brain tissue during the space flight. Therefore, the goal of this study was to examine the long-term exposure to microgravity on β-endorphin receptor concentration in a variety of rat brain regions.
3. Methods
The experiment was conducted in the laboratory animal center and in compliance with the recommendations of the animal care committee of the AJA University of Medical Sciences, Tehran, Iran. All procedures used in this study were conformed to the national research council (NRC) guide for the care and use of laboratory animals. A total of 16 randomly selected adult male Sprague-Dawley rats (250 - 320 g body weight) were housed in individual cages under controlled temperature (22°C), humidity (∼40%), and lighting (12 - 12 h light-dark cycle) conditions, with free access to food and water. The rats were divided into two groups (n = 8/group) of freely moving (control group) and hindlimb unloading rats. According to the method of Morey- Holton, we used tail suspension model to simulate microgravity (13, 14). The rats in the experiment groups were suspended by the tail base to produce a 35 - 45° head-down tilt position allowing the forelimbs to maintain contact with the grid floor and the rat to move in a circular area to gain access to food and water for 14 days.
After 14 days, the rats were fasted overnight, anesthetized with intraperitoneal injection of ketamine and xylazin (100 and 2.5 mg/kg, respectively), and killed by exsanguinations. The entire brain was removed and was segmented into brain stem, prefrontal cortex, and hippocampus. Each sample was weighed, washed in ice-cold NaCl solution (9 g/L), and then immediately frozen in liquid nitrogen. Tissues were homogenized in Tris-HCl buffer, pH 7.4 (tissue to buffer ratio, 1:10 w/v). The homogenate was centrifuged (10 min, 14000 x g, 4°C). The supernatant was diluted further (1:10, v/v) in Tris HCl buffer. Supernatants were obtained after centrifugation. β-endorphin receptor concentration was analyzed by ELISA technology using commercially available kits (CUSABIO, Wuhan, China), according to the manufacturers’ instruction, which provided for tissue homogenates, cell lysates, and other biological fluids samples.
Statistical analyses were performed using SPSS v16. All data are presented as means ± SEM. Statistical analyses were performed using un-paired Student’s t-test for comparisons. For all analyses, P < 0.05 was considered statistically significant.
4. Results
The mean level of µ-receptor (ρg/100mg tissue) was 1700 ± 320 and 2743 ± 166 in the brain stem of freely moving and hindlimb unloading rats, respectively (P < 0.05; Figure 1).
The mean µ-receptor level of prefrontal cortex in the hindlimb unloading rats (1410 ± 160) was significantly higher than in the freely moving rats (743 ± 110) (P < 0.05; Figure 1).
There was no significant difference in the mean µ-receptor level of hippocampus between the hindlimb unloading rats (902 ± 77) and the freely moving rats (667 ± 88) (P > 0.05; Figure 1).
5. Discussion
Endocrine system appears to be sensitive to the conditions of space flight including microgravity. During space flights, most astronauts report a moderate to severe headache due to cephalic fluid shifts (15). Fluid shifts itself cause cranial venous congestion (16). β-endorphin exerts an inhibitory regulation on the inflammatory responses through the activation of µ-opioid receptors (17). β- endorphin also induces vasoconstriction in CNS (18). In this study, the β-endorphin receptor level in the brain stem and prefrontal cortex upregulated in the simulated microgravity condition. The probable mechanism is as follow. During space flight and simulator conditions several stress factors such as microgravity can affect the function of neuroendocrine system. Plasma levels of cortisol were markedly increased in rats exposed to space flight (19). It has been shown that cortisol inhibits β-endorphin secretion (20). It seems that following exposure to stress of unloading, cortisol levels increase, which reduces β-endorphin, which in turn upregulate its receptor.
Cortisol also counteracts insulin, contributes to hyperglycemia-causing hepatic gluconeogenesis and inhibits the peripheral use of glucose that can cause insulin resistance (21). Glucocorticoids impair insulin-mediated glucose uptake by directly interfering with components of the insulin-signaling cascade, such as glycogen synthase kinase-3, glycogen synthase, and especially GLUT4 translocation (22, 23). In a long period, the increasing of cortisol can cause hyperglycemia. In other words, insulin sensitivity is reduced in astronauts during space flight and microgravity condition (24).
Opioids play an important role in the regulation of glucose homeostasis (25). It is possible that hypercortisolemia and hyperglycemia, which are induced by microgravity, can increase β-endorphin receptor concentration. This study confirms one previous study on opioid mu-receptor that showed that hyperglycemia was responsible for the increase of opioid mu-receptor in STZ-diabetic rats (26).
It is shown that μ opioid receptor is involved in the homeostatic regulation of immunological and inflammatory reactions in the mouse gastrointestinal tract. The µ opioid receptor gene expression is increased during intestinal inflammation (27). Increased migration of monocytes and lymphocytes to the inflamed intestinal mucosa are the major cause. Inflammation is often associated with enhancement of μ opioid receptor axonal transport (26, 27). During microgravity condition, activation of T lymphocytes and inflammatory cytokines happened (28). Beta-endorphins can reduce the inflammatory response associated with the µ receptor (29). The alteration in β-endorphin receptor concentration may also be due to this reason.
5.1. Conclusion
It is possible that simulated microgravity may increase the β-endorphin receptor expression in the prefrontal cortex and brain stem in a rat.