1. Background
Teucrium polium (TP), a plant with medicinal properties from Lamiaceae family has long been used in Iranian traditional medicine for various purposes such as diabetes, diarrhea, abdominal tension, pain, inflammation, bacterial infection, convulsion, and dementia. The plant is vastly distributed in countries such as Armenia, Spain, Algeria, Tunisia, Turkey, Syria, Iraq, and Iran. Recent studies have confirmed wide ranges of diseases that can be affected by plant use, including cancer, gastric disorders, visceral pain, diabetes, inflammation, infectious states, vascular disorders, hepatic disease, rheumatism, and central nervous system-related diseases (1, 2). TP has potent antioxidant, anti-choline esterase, anti-inflammatory, anti-apoptotic, and hippocampal neuronal survival properties (3, 4). These multi-purpose effects can logically be designed for various debilitating central nervous system-related diseases such as AD.
2. Objectives
In this study, we used daily intraperitoneal (IP) single-dose injection of TP (3 mg/kg) taken from a preliminary study for a week to show its memory enhancing effects on spatial memory performance and CA1 neuronal counts in rat Aβ25-35 model of AD.
3. Methods
3.1. Animals
Thirty male Wistar rats aged 10 - 12 weeks and weighing 230 - 240 g equally divided into three groups as follows: In the group 1 (sham), AD was induced by bilateral intrahippocampal microinjections of a single dose of 10 µg/2 µL Aβ25-35 (Aβ). This is the dose which is used by previous investigators (5). Animals in this group received additional daily IP injection of 2 mL normal saline for a week. In the group 2 (control), animals received daily IP injection of TP (3 mg/kg dissolved in normal saline) for a week. This dose has been taken from our previous study, which will appear in a manuscript under publication (6). In the group 3 (treated), AD was induced by bilateral intrahippocampal microinjections of single 10 µg/2 µL dose of Aβ25-35 (Aβ) followed by daily IP injection of TP (3 mg/kg dissolved in normal saline) for a week. Rats were kept in plexiglass cages in the Department of Physiology, Tehran University of Medical Sciences, at a constant temperature and humidity with free access to water and standard chow in a 12-hour light dark cycle. Procedures for care and animal handling meticulously followed in accordance with Tehran University Ethical Committee protocol (IR.TUMS.VCR.REC. No. 1395.1392) and National Institute of Health (NIH) recommendations (7).
3.2. Drugs
Plant extract preparation. TP Lamiaceae plant was gathered from Alborz mountains, 30 km northwest of Tehran during spring. The aerial parts of the plant were dried at ambient temperature and ground in a mortar to make a powder form. To make the hydro-alcoholic extract, 2000 g of the prepared material was macerated in ethanol and water (75/25 v/v) for 72 hours at room temperature. The procedure was repeated 48 and 24 hours for further evaporation. The final remnants were filtered with Whatman paper and kept in an oven for 36 hours at 55°C. Herbarium samples were kept on record No. PMP-387 in pharmaceutical faculty, taxonomy department of Tehran University of Medical Sciences.
3.3. Amyloid Beta (Aβ)
The chemical was purchased from Sigma Aldrich CHEMIE GmbH (Riedstra Steinheim Germany). Two microliters of pre-aggregated Aβ25-35 (5 µg/µL) solution were dissolved in normal saline (pH = 8, pre-incubated for 72 hours at 37°C) and kept at -80°C before use.
3.4. Experimental Procedure
The spatial MWM memory test was carried out from 8 a.m. till 4 p.m.
Animals were acclimatized for a week in the new environment and initially checked for health status, visual, locomotive, tactile, and auditory capabilities. After general anesthesia with IP co-injection of ketamine (75 mg/kg) and xylazine (10 mg/kg), stereotaxic surgery was performed. The animal’s head was symmetrically put in the apparatus to have an exact skull flat head position. Head skin was shaved and disinfected with a cotton bullet soaked in the povidone iodine 10% solution. The scalp was cut in the midline position to expose the skull sutures
We inserted two needle bur on animal’s scalp in streotaxic method at coordinates of -3.5 mm posterior to the bregma, ± 2 mm lateral to sagittal suture, and 2.8 mm ventral to dura matter (8) for infusion of Aβ25-35 solution.Two microliters of pre-aggregated Aβ25-35 (5 µg/µL) solution was dissolved in normal saline (pH = 8, pre-incubated for 72 hours at 37°C) and bilaterally injected in the dorsal hippocampus CA1 region to induce AD. After a two-week surgical recovery, TP dissolved in normal saline at 7.4 pH and was IP injected once a day for a week.
Animal’s body weight was monitored weekly for a suitable TP dose adjustment. Spatial MWM performance was tested an hour post last TP dose on day 21st started from the first study date by the observer blinded to the test outcomes. In spatial MWM memory test, we followed the same protocol in the previous study (9). The test started on day 21 till day 27 post AD induction in a circular black pool (150 cm diameter, 60 cm height) filled with tap water and installed in the room at ambient temperature. The water tank divided equally into four quadrants. A transparent circular small hidden platform submerged 1 cm under the water and 20 cm away from the tank wall in the southeast quadrant. Conspicuous English letters were mounted in the room walls as the visual landmarks for the animal platform location navigation.
On day 21, a training memory session held in both dark and illuminated room with aluminum foil covered and bare platform first. The animals were then wiped out with a clean and dry fabric and returned to their respective cages for the reference memory task test an hour later. The test was repeated each day for five subsequent days in which the animal smoothly was put into the water from a random quadrant while his face turning into the wall for four 60 second trials each day with a 30 second within trial rest. A mounted digital camera connected to the computer setting equipped with the navigation recording software (Radiab software 2.1, Nomirei Co., Tehran Iran) monitored the swimming animal from the ceiling. Time, velocity, distance, and path track in different quadrants were all recorded and processed. On day 26, probe test was carried out to measure the reference memory through removal of the hidden platform and allow the animals to start swimming from the farthest point in the water tank for 60 seconds. Time spent in the target quadrant, path track, goal, and swimming distance were all then recorded and compared in different study groups.
3.5. Histological Exam
This method of staining is used to identify Nissl substance in the cytoplasm of neurons in paraformaldehyde-fixed tissue sections. The Nissl bodies will be stained in purple-blue granular hues. To prepare the stain, 0.1 g Cresyl violet powder dissolved in 100 mL distilled water (0.1% solution) at first. Then 10 drops glacial acetic acid was added to the solution and filtered. Gelatin coated slides with hippocampal sections in different study groups were mounted on special tissue racks and immersed in ethanol 100% for 5 minutes for defatting the slides’ surfaces. The slides put in distilled water for 15 minutes first and floated in prepared Cresyl violet solution. The slides were immersed in distilled water again and removed for three times and put into ethanol 95% for 2 minutes. Final dehydration was done via immersion of the slides into the ethanol 100% for 2 minutes. Eventually, the slides were placed in xylene solution for 10 minutes and removed for air dry at ambient temperature. Stained slides were covered with cover slips using Entellan medium for light microscopic observation (10).
3.6. Statistical Analysis
Data were presented as mean ± SEM. Two-way repeated measure ANOVA for MWM escape through latency and swum distance, one-way ANOVA for probe test, and neuronal count with Bonferroni as post hoc were used in GraphPad Prism statistical software version 6. The P < 0.05 was considered statistically significant. We have explored the statistical significance between Aβ-received TP-treated (treated) group compared to AD induced (sham) and TP-received (control) animals.
4. Results
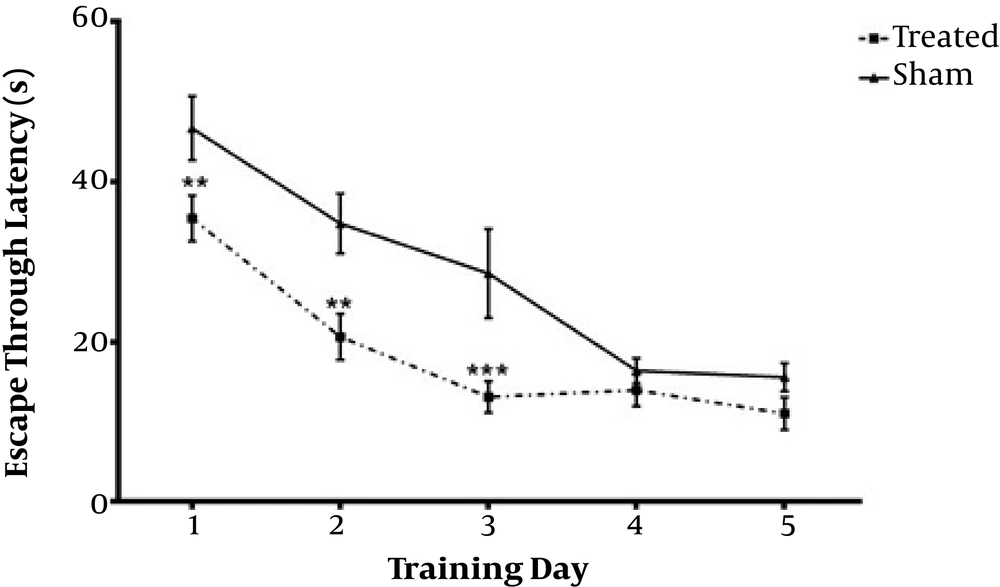
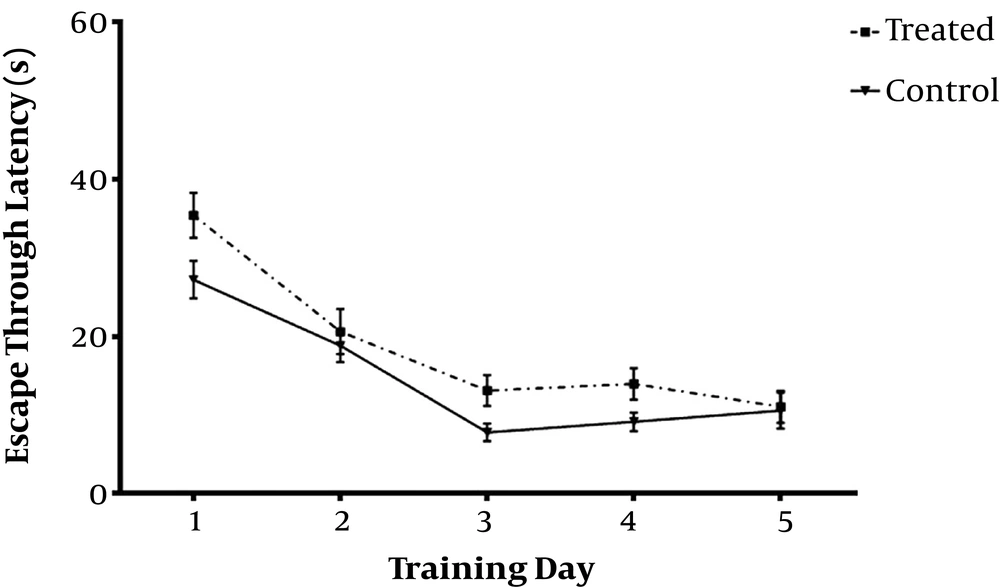
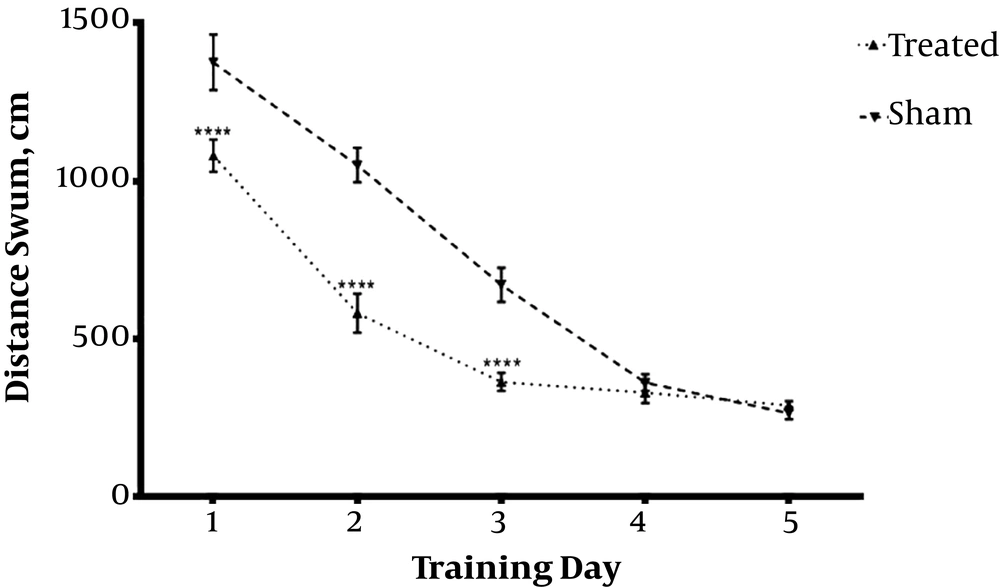
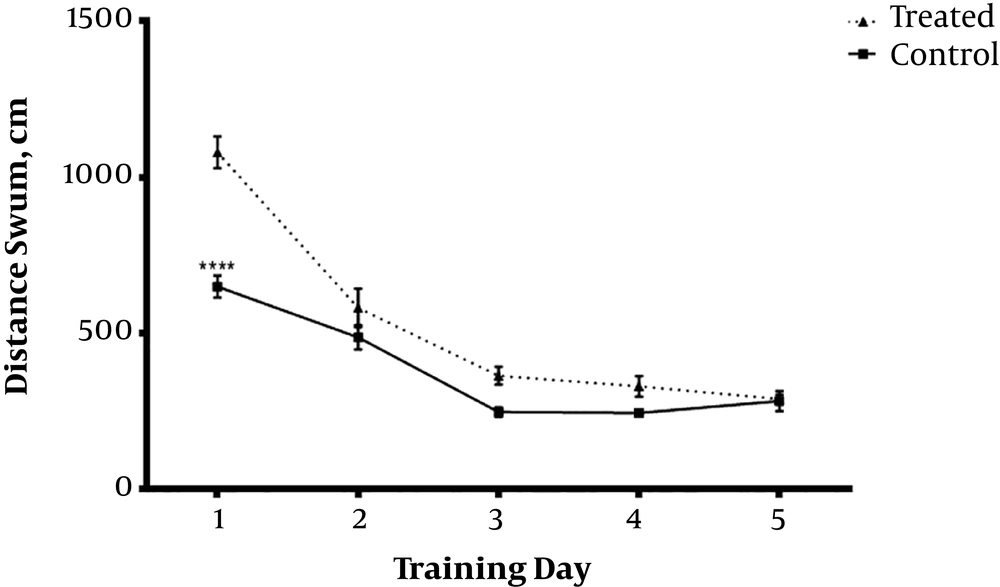
Daily IP administration of 3 mg/kg TP as hydro-alcoholic extract for one week in Aβ-received animals (Treated) group significantly decreased escape through latency (Figures 1 and 2) and distance swum (Figures 3 and 4) till the 4th day compared with Aβ- (sham) and TP-received (control) groups. F(8,72) = 16.94, P < 0.0001 F(8,72) = 1.747, P = 0.1022 respectively. The significant difference is shown by respective asterix.
Animals received bilateral intrahippocampal microinjections of a single dose of (10 µg/2 µL) Aβ25-35 followed by one week additional daily intraperitoneal injection of 2 µL normal saline (sham); and bilateral intrahippocampal microinjections of Aβ25-35 (10 µg/2 µL) followed by one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (treated). Data are presented as mean ± SEM. ** Significantly different from Aβ-treated group at P < 0.01, *** significantly different from Aβ-treated group at P < 0.001 using repeated measures two-way ANOVA.
Animals received one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (control); and bilateral intrahippocampal microinjections of Aβ25-35 (10 µg/2 µL) followed by one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (treated). Data are presented as mean ± SEM and analyzed using repeated measures two-way ANOVA.
Animals received bilateral intrahippocampal microinjections of a single dose of (10 µg/2 µL) Aβ25-35 followed by one week additional daily intraperitoneal injection of 2 µL normal saline (sham); and bilateral intrahippocampal microinjections of Aβ25-35 (10 µg/2 µL) followed by one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (treated). Data are presented as mean ± SEM. **** Significantly different from Aβ-treated group at P < 0.0001 using repeated measures two-way ANOVA.
Animals received one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (control); and bilateral intrahippocampal microinjections of Aβ25-35 (10 µg/2 µL) followed by one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (treated). Data presented as mean ± SEM. **** Significantly different from Aβ group at P < 0.0001 using repeated measures two-way ANOVA.
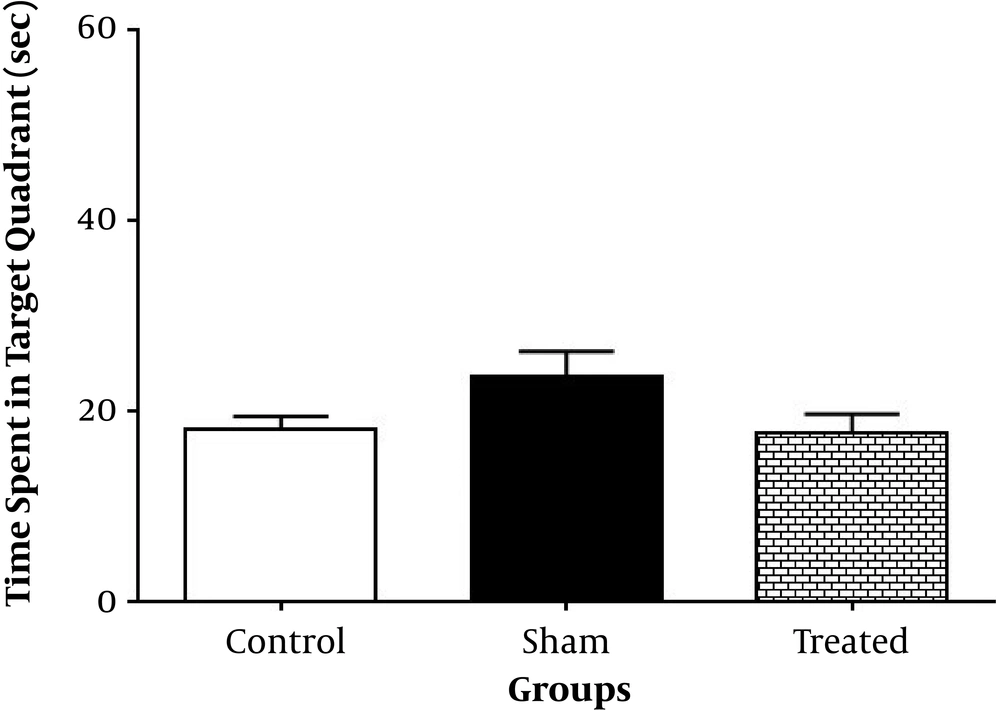
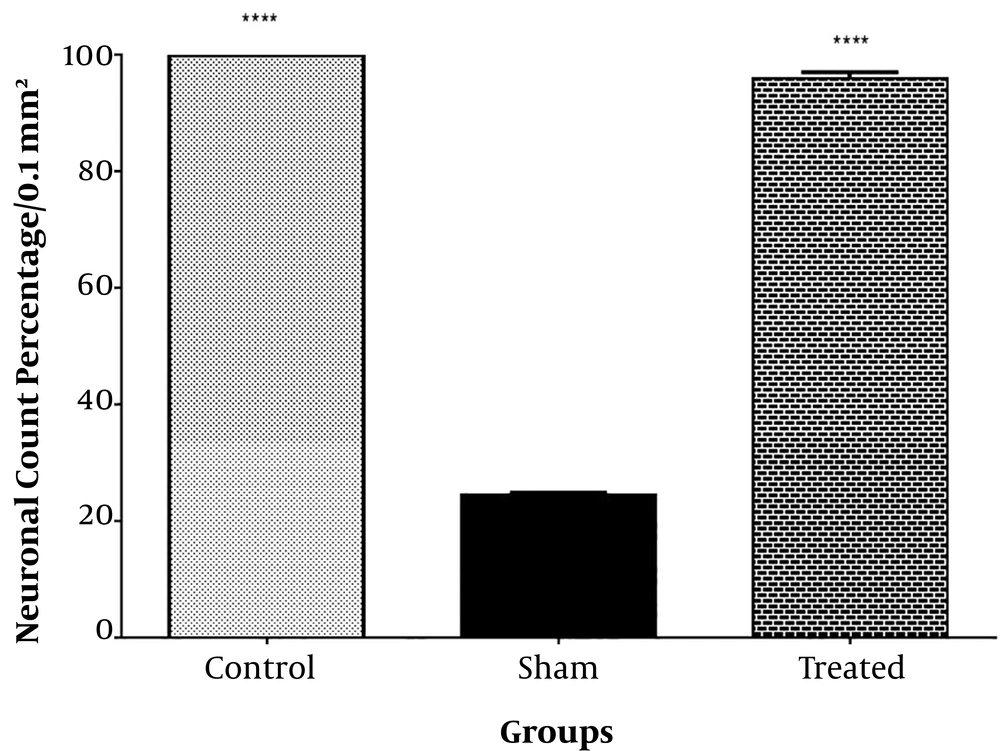
Time spent in target quadrant with removed hidden platform on day 26 showed no statistical significant changes between the treated group compared to the sham and control groups (P > 0.05, n = 10) (Figure 5). The intact neuronal count percentage of the CA1 hippocampal region, however, showed a significant rise in the treated group compared to the sham (P < 0.0001, n = 3) and control groups (P < 0.0001, n = 3) (Figures 6 and 7).
Animals received bilateral intrahippocampal microinjections of a single dose of (10 µg/2 µL) Aβ25-35 followed by one week additional daily intraperitoneal injection of 2 µL normal saline (sham); one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (control); and bilateral intrahippocampal microinjections of Aβ25-35 (10 µg/2 µL) followed by one week intraperitoneal hydroalcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (treated). Data are presented as mean ± SEM.
Animals received bilateral intrahippocampal microinjections of a single dose of (10 µg/2 µL) Aβ25-35 followed by one week additional daily intraperitoneal injection of 2 µL normal saline (sham); one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (control); and bilateral intrahippocampal microinjections of Aβ25-35 (10 µg/2 µL) followed by one week intraperitoneal hydro-alcoholic extract of 3 mg/kg Teucrium polium dissolved in normal saline (treated). Data are presented as mean ± SEM. **** Significantly different from Aβ-treated group at P < 0.0001 using one-way ANOVA.
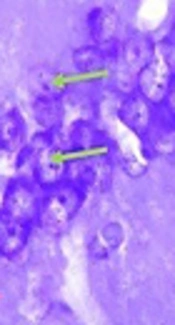
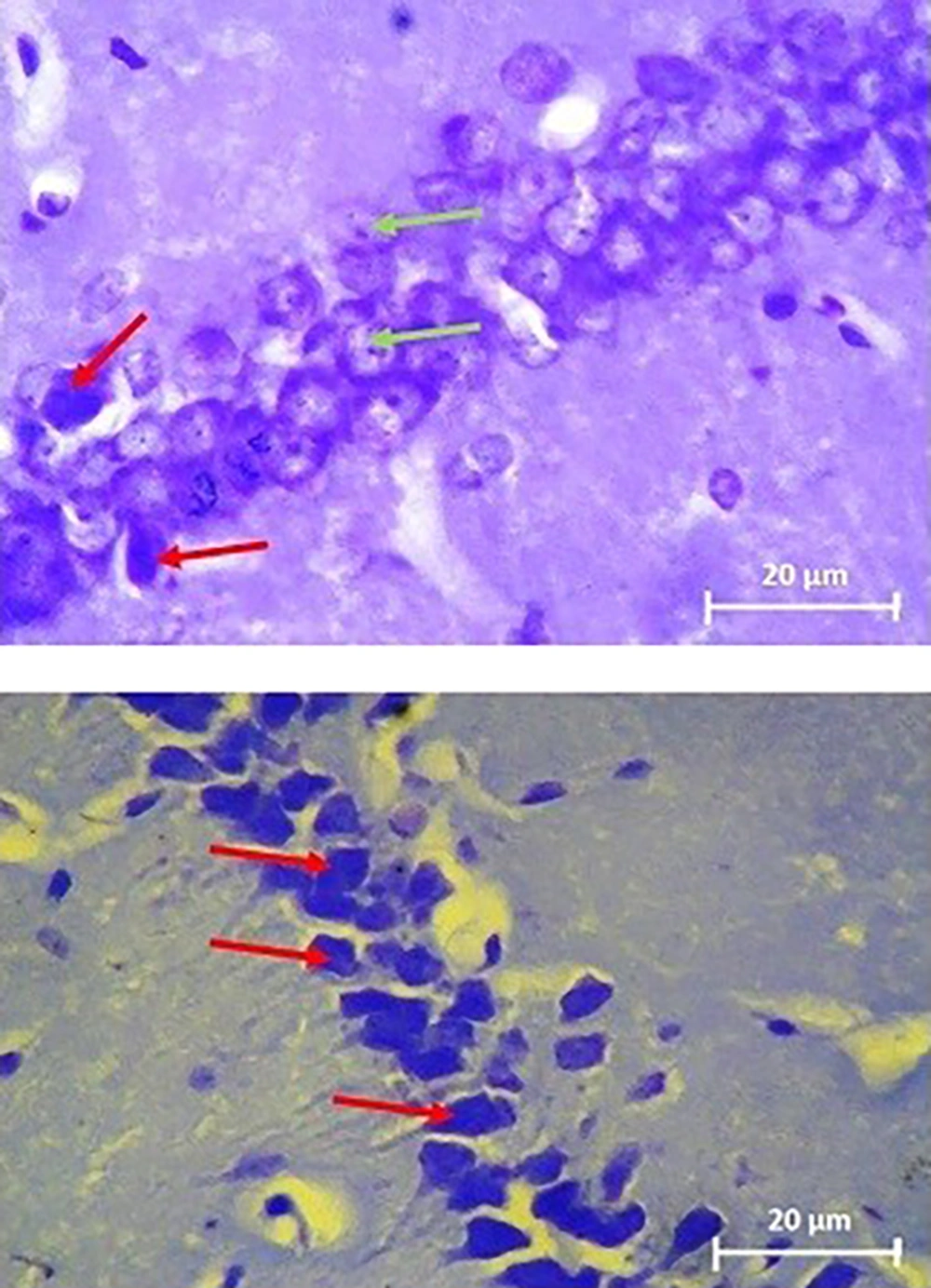
Neuronal architecture in CA1 region of hippocampus stained with Cresyl violet in bilateral intrahippocampal microinjections of Aβ 25-35 animals (sham) (above) and daily intraperitoneal administration of 3 mg/kg hydro-alcoholic extract of Teucrium polium treatment for one week in Aβ-received animals (treated) (below). The images were taken with ×400 magnifications. Aβ-received animals (sham) had much less cell density, which is indicative of more dead neurons compared to Teucrium polium-treated animals (treated). Note that cells with black cytoplasmic color with destructed nucleus and unmarked membrane are indicative of total disorganized cells in Aβ-received animals (above). Cells with bright blue or grey cytoplasmic color with round notable membrane and nuclear architecture can be identified in Teucrium polium-treated animals (treated). Green and red arrows are representative of normal and dead cells, respectively. Scale bars: 20 µm.
5. Discussion
The present study shows that very low dose (3 mg/kg) daily intraperitoneal injection of TP in the form of hydro-alcoholic extract for a week in AD-induced animals (treated) reduces escape through latency and distance swum in spatial memory performance. Moreover, we have observed an increase in live neuronal density in CA1 region of the hippocampus compared with sham Aβ-received group. This may be the first in vivo report for memory enhancing effect and neuronal survival properties of the plant at this very low dose. Although there is controversy on the safety reputation of TP (11-13), our previous dose response study of TP documented its safety at very low doses (3, 10, 30 mg/kg) vs. high doses (200 and 400 mg/kg) (6).
Bilateral intrahippocampal injections of aggregated Aβ25-35 have shown to produce MWM performance deficit (9, 14). In the present study, escape latency and distance swum showed a comparable increase till the fourth training day and reached the level of “only TP-treated” (control) animals. Different factors, which may play a part in this response, are suggested to be TP dose and its route of administration, spatial memory test length, Aβ fragment used (15), time lapse between MWM test, and histological assays. Additional factors such as neuronal migration, regeneration and hypertrophy and even intrahippocampal regional response differences to the toxic fragment (16), MWM stimulatory effect on memory and learning (17), and possible recovery phenomenon (14) should be added to the list. Time spent in target quadrant showed no significant changes in probe reference test of different study groups on day 26. This can be rationalized through nano-molar toxic concentration to pico-molar protective change with time due to recovery or compensatory phenomenon. Protective change maybe due to neuronal migration from subgranular zones and neurogenesis in cortical regions (17) together with MWM training days induced dentate gyrus neurogenesis should also be responsible (18).
MWM spatial memory under performance linked with neuronal loss in the CA1 hippocampal region in Aβ-received rats is confirmatory evidence for neurotoxicity of the material and ensure the accuracy of the rat AD model used in the present study. However, escape through latency and distance swum curve slopes and also probe test result comparisons between study groups show a better performance in Aβ-received rat model of AD than control and treated animals as mentioned earlier in other works (19). Significant neuronal loss in Aβ-received animals (sham group), suggested to be induced via different mechanisms such as neuroinflammation (20), neurodegeneration, apoptosis (15), oxidative stress, acetylcholine esterase activation (14), synaptic plasticity, and dendritic changes (21, 22).
Different researchers have suggested that the effects of this plant extract might be due to one or several of its active ingredients such as flavonoid, terpenoid, iridoid, and phenilpropanoid glycosides (23), tannin, saponin, sterol, β-caryophyllene, and diterpenoids (24, 25). The ingredients vastly differ based on the meteorological condition, land physical and chemical characteristics, used plant tissues, plant age, developmental phase, and harvesting time. Many other active compounds with potential phytomedicine properties, especially phytoestrogens based on geographical distribution of the plant (2). Phytoestrogens that have been found in the plant are plant origin estrogen-like compounds that mimic neuroprotective effects of endogenous estrogen with the least untoward sequels via its alpha and beta receptors distributed specifically in CA3 intrahippocampal region with potential estrogen receptor mediated cell survival signaling and memory enhancing responses (4).
5.1. Conclusions
Despite remarkable literature findings against TP toxic effects, recent reports have demonstrated potential therapeutic properties of the plant and its active ingredients. Further studies will clarify the possible beneficial use of the plant extract in Alzheimer’s disease.