1. Background
Various characteristics and intensity of stimuli have different effects on learning and memory. Physical exercise and sleep can shift the learning and memory quality by affecting intracellular factors and the expression of different neurotransmitters that mediate this ability (1). Varies traits of physical exercise determine the exercise-induced effects; such as the different form of exercises like aerobics or resistance, the frequency of repetition, the intensity and volume of exercise (2).
In light of the evidence from different examines, we have a better understanding that moderate intensity of activity might improve cognitive functions (3), mood (4), increased plasticity in young and adult (5), learning and memory (6), and executive function (7). Whereas the detrimental effects of severe intensity of physical exercise on brain were proved; like mitochondrial dysfunction (8), changes in intracellular cascades (9), and disruption of hippocampal neurogenesis (10); moreover, intensive and exhaustive running programs resulting to impaired hippocampus-dependent memory due to a high index of oxidative stress (11).
Several recent studies have demonstrated the importance of functional interactions between the areas involved in learning and memory like hippocampus and prefrontal cortex. These interactions are crucial for the incorporation of cognitive and emotional information for supporting adaptive actions like spatial information (12).
Multiple factors like physical exercise (13) or amount of sleep (14) can impact on the neurotransmissions and cognitive capacity. Animal studies suggest that insufficient sleep increases level of stress hormones, which may reduce the new cell production in adult brains (15), neuroplasticity (16), proliferation of hippocampus cells (17), inhibiting mitochondrial metabolism (18), protein synthesis pathways (19), induce cellular and molecular inflammatory variations (20), and increased Aβ plaque formation (21).
Mammalian target of rapamycin (mTOR) is a protein kinase involved in the synaptic plasticity and memory and its pathway is activated during various cellular processes. Brain neurotrophic factors, amino acids, inflammation, and glucose are some of triggers of this cascade. This factor was used in animal studies for evaluating the effect of different factors on learning and memory (22).
There are different intracellular signaling cascades, which regulate learning and memory; MAPK, PI-3 kinase, PLC, mTOR pathway, and etc. (23). mTOR is a protein kinase involved in translation control, long-lasting synaptic plasticity, and memory.
There have been a number of studies that show that sleep deprivation influences adversely on cognitive abilities like both non-declarative and declarative learning and memory (24, 25), attention (26), decision-making (27), and emotion perception (28). Moreover, some literature has emerged contradictory findings of the effect of sleep deprivation on learning and memory. Different types of SD, either total or partial, have a fast and transient antidepressant effect (29) and they were considered as a short-term treatment for stress-induced depressive behaviors (29).
2. Objectives
In this research, we evaluate the amount of mTOR in the hippocampus and prefrontal cortex to examine the effect of REM sleep deprivation (REM-SD) on the memory impairment induced by severe physical exercise.
3. Methods
3.1. Animals
Adult male, Wistar rats were housed in groups of four per cage on a 12 hours light: 12 hours dark cycle with free access to the food and water. The experimental protocols and procedures in this study were approved by the Research and Ethics Committee of Tehran University of Medical Sciences (NIH publications No. 80-23; revised 1996).
3.2. Experimental Procedure
Animals were randomly distributed into four different groups, control (Cont.), physical exercise (Ex), 24 hours of REM-sleep deprivation (24REM-SD), and the intervention of EX and 24REM-SD (ExSd). Control animals are put in the immobile treadmill as long as the Ex group. Animals in the intervention group were gone under 24 hours REM-SD after the last session of exercise. The Ex group received five weeks of treadmill exercise with increasing intensity gradually. Animals were sacrificed and the brain tissue was collected for assessing the amount of mTOR protein expression.
3.3. Exercise Training Protocol
Using a 4-lane animal treadmill (IITC Life Science Inc., USA), rats were handled and trained to familiarize with running. The bars at the end of the treadmill delivered electrical shocks (1.0 mA) to the hind part of the body when the animal stops running and forced them to run forward during exercise. After two days of rest, the procedure was performed, one session daily, five days a week for five weeks. The main phase of treadmill training started at a speed of 18 m/min for 30 minutes and no tilt for the first week. The time duration and tilt increased progressively, 10 minutes and five degrees increased each week. The speed was maintained at 18m/min for the entire period. Sedentary animals were placed on a stationary treadmill daily and were given electrical stimulation in a way that was identical to that used for the exercise group.
3.4. Induction of REM Sleep Deprivation
REM sleep deprivation was induced by placing four to six rats in modified water-filled multiple platforms that consisted of eight small circular platforms, which submerged to about 1 - 2 cm above the water surface. The platforms were located at a distance so rats could move freely from one to another and balance on the platforms. Due to natural REM’s muscle paralysis, the rats would be exposed to the water and awaken. The rats had free access to clean water and food pellet baskets hanging from the aquarium cover. For the control group, using larger platforms caused falling asleep without falling down. The REM sleep deprivation period lasted for 24 hours.
3.5. Western Blotting
Protein concentration was measured by spectrophotometry at 230 nm using Picodrop instrument (Picodrop, Hinxton, UK), and the results were acquired as milligram per milliliter. A total of 60 microgram of each sample was loaded in SDS-8% polyacrylamide gel (pH = 8.3) and electrophoresis was carried out at 120 V for 120 minutes, then, they were transferred to polyvinylidene fluoride (PVDF) membrane (Chemicon Millipore Co. Temecula, USA). To block non-specific protein binding sites, membranes were incubated in 5% skim milk (pH = 8.6) for 90 minutes. Then, blots were incubated overnight at 4°C with a primary antibody (mTOR and p-mTOR primary antibody, Abcam, 1:1000 diluted in skimmed milk). On the next day, Tris-buffered saline and Tween 20 (TBST) were used to wash membranes three times and then blots were incubated for 1 hour, with a secondary antibody (Horseradish peroxidase-linked goat anti-rabbit IgG, Abcam, 1:5000). To detect bounds, blots were exposed by enhanced chemiluminescence (ECL; Amersham, UK) and then visualized by exposure to autoradiographic film for the adequate time.
3.6. Statistical Analysis
Statistical Package for the social sciences (SPSS, version 23) was used for this study. For analyzing western blotting data, Image J software was used to densitometry of bonds. After that, data obtained with Image J were subjected to one-way ANOVA analysis using SPSS. Post-hoc (Tukey) test was used for within-subject comparisons. In all comparisons, the P values 0.05 and less were considered as statistically significant.
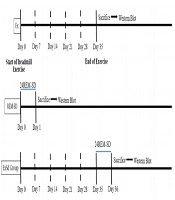
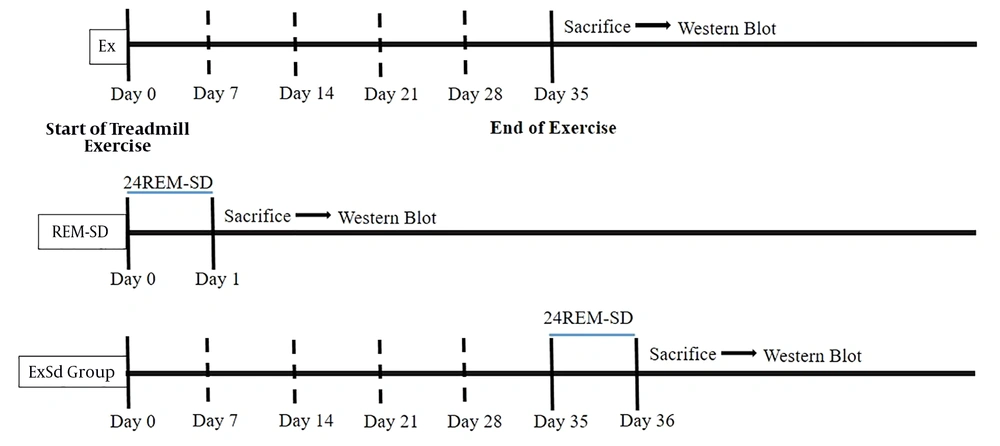
The entire experimental procedure part is summarized as a timetable in Figure 1.
The schematic timeline for study design. Two groups of animals received five weeks of physical exercise with a gradual increase in intensity (5 weeks, 5 days in a week). Animals in ExSd group was under gone 24 hours REM-SD after the last session of exercise. REM-SD just received 24h REM-sleep deprivation. Control animals received nothing. All animals were sacrificed after experimental procedures and their brain was collected for western blotting test.
4. Results
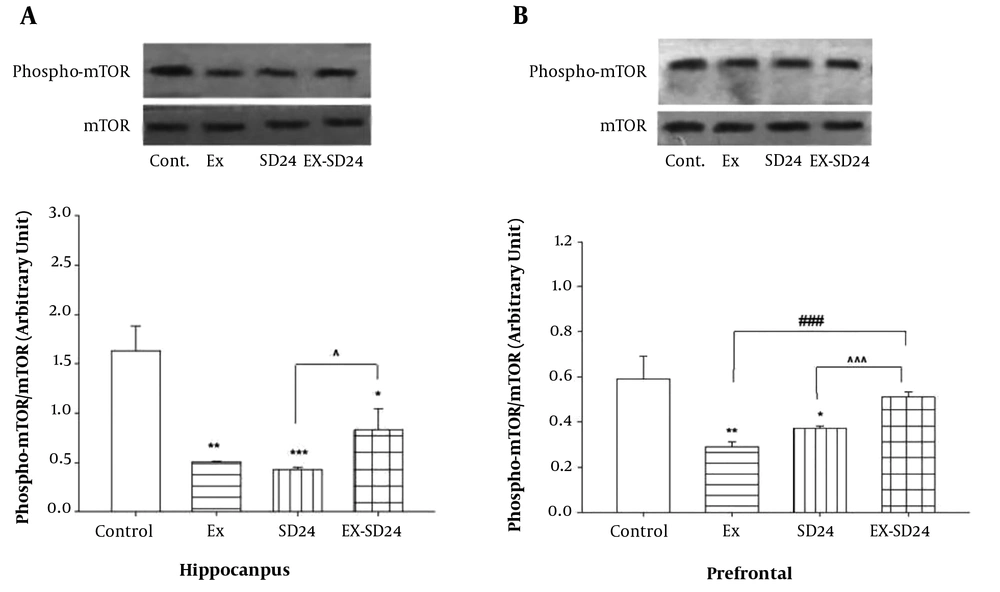
4.1. The Effect of the Combination of High-Intensity Exercise and REM-Sleep Deprivation on Hippocampal p-mTOR Levels
Figure 2A shows that there is a significant difference between groups versus control in p-mTOR levels in the hippocampus [F (3,16) = 11.06, P < 0.0001]. Post-hoc Tukey analysis showed that there were significant differences in the level of p-mTOR in the hippocampus between the Ex (P < 0.01), SD (P < 0.001), and ExSd (P < 0.05) groups as compared with the control group. In addition, ExSd group showed an enhancement in p-mTOR level in comparison to the SD (P < 0.05) groups in the hippocampus.
A, The effect of 24h REM-SD on the decreased level of mTOR in severe exercised group in the hippocampus of the rat. Values are expressed as the means ± S.E.M. (n = 5 for each group); *P < 0.05, **P < 0.01 and ***P < 0.001 versus control, ^P < 0.05 versus SD24. Ex = exercised group; SD24 = 24h REM- sleep deprivation; ExSd = they gave REM-SD after five weeks intensive exercise. B, the effect of 24h REM-SD on the decreased level of mTOR in severe exercised group in the prefrontal cortex of the rat. Values are expressed as the means ± S.E.M. (n = 5 for each group); *P < 0.05, **P < 0.01 versus control, ^^^P < 0.001 versus SD24 and ###P < 0.001 versus Ex. Ex = exercised group; SD24 = 24h REM- sleep deprivation; ExSd = they gave REM-SD after five weeks intensive exercise.
4.2. The Effect of the Combination of High-Intensity Exercise and REM-Sleep Deprivation on Prefrontal p-mTOR Levels
Figure 2B illustrates that there is a significant difference between groups in p-mTOR levels in the prefrontal cortex [F (3,16) = 7.01, P < 0.001]. Post-hoc Tukey analysis showed p-mTOR level in the PFC is significantly lower in the Ex (P < 0.01) and SD (P < 0.05) groups when compared with the control group; however. There is no significant difference between ExSd and the control group. Following the intervention of Ex and SD, phosphorylation of mTOR was elevated when compared with the Ex and SD (P < 0.001) groups.
5. Discussion
The health benefits of a moderate physical exercise are accepted, however, severe physical exercises lead to various adverse effects in the brain and body. such as physical fatigue and exhaustion (3), excessive blood lactate (30), decrease in glucose uptake (31, 32), changes in metabolism (33), oxidative stress (34, 35), alteration of inflammatory cytokine expression (36), and increasing the susceptibility to infection (37). Moreover, stress might be the most detrimental factor, which can increase the concentration of reactive oxygen species (ROS) (38). Brain with high mitochondrial energy metabolism and weak antioxidant defense could be most affected by oxidative damage induced by high ROS levels (39). It results in reduction in the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway in the PFC (40), reduction in phosphorylation levels of mTOR and phospho-p70S6K in the PFC and hippocampus (41, 42) or decreases BDNF expression in parallel to reduced phosphorylation of mTOR and p70S6K in the hippocampus of rats (43).
In the current study, the combined effect of SD and intensive exercise on the level of p-mTOR -as a marker of learning and memory- in the hippocampus and PFC was investigated. Our previous study indicated that severe exercise causes a decrease of the total time in the target zone in the Morris Water Maze test with corresponding reductions in the expression of the hippocampal and prefrontal level of BDNF and TrkB, which reduce memory performance (under review).
The experiments that were carried out throughout sleep deprivation showed controversial results. It is generally accepted that sleep deprivation, mostly, has a negative effect on learning and memory by increasing hippocampal oxidative stress (44), reduction of functional NMDAR in the hippocampal neurons (45), attenuating mTOR-dependent protein synthesis (46), decreases levels of brain-derived neurotrophic factor (BDNF), and phosphorylated-cAMP response element-binding protein (P-CREB) (47). However, in glaring contradiction to the general view, some findings indicated different actions of sleep deprivation as an experimental model of antidepressant treatment (48) and mood improvement (49). In addition, short SD enhanced BDNF level in the hippocampus has been shown (50). Furthermore, one night of sleep deprivation significantly increased cell proliferation in the hippocampus of rat (51). There is a study that indicated that the extended wakefulness might result in an increased level of BDNF in the hippocampus (52).
To date, the mechanism of this controversial effect of sleep deprivation has not been clarified. Sei et al. (53) showed that there is no significant change in the BDNF level in the rat hippocampus under the minimized stressful condition. We proposed that sleep deprivation might act as a compensatory factor only when the animal is under severe stressful condition. To the best of our knowledge, it is the first study that evaluated the level of p-mTOR in the SD animals.
5.1. Conclusions
The evidence from this study suggests that severe exercise and REM-SD decreased the p-mTOR level in the PFC and hippocampus. As mTOR had a critical role in cell proliferation, transcription, and cell growth, the deleterious effect of sever-exercise and REM-SD might be related to this kinase. Our results showed that 24 h REM-SD after five weeks of sever exercise increased the level of p-mTOR in the PFC and hippocampus, which might be related to the dual effect of SD on the cognitive function.