1. Introduction
chronic granulomatous disease (CGD) is a genetic disease caused by a defect in the NADPH oxidase complex that constitutes phagocytic oxidase (Phox). This defect causes phagocytes to be incapable of germ elimination. Clinically, this defect is characterized by recurrent life-threatening bacterial and fungal infections. The chronic granulomatous disease occurs in one out of 200,000 live births, usually before the age of five. Common infections in CGD are pneumonia, skin and organ abscesses, purulent adenitis, bacteremia, fungemia, osteomyelitis, and cellulite (1).
Pericardial effusion occurs due to fluid accumulation more than the normal physiological state in the fibroelastic sac of the pericardium. Pericardial effusion can be caused by acute infectious (TB, pyogenic, viral), idiopathic, or autoimmune (SLE, JIA) diseases, uremia, cardiac surgery manipulations, chest trauma, malignancies, renal failure, and ascending aortic dissection (2, 3). Pleural effusion is more common in children, due to bacterial, viral, and fungal infections, rheumatic and cardiovascular disease, renal trauma, and malignancy (4, 5). Concomitant pleural and pericardial effusion is reported in bacterial infection, autoimmune disease, and malignant condition. Pericardial effusion is seen in 52% of patients with empyema (6). Solitary pericardial involvement is infrequent in CGD and rarely reported in case studies (7, 8). Concomitant pleural and pericardial effusion is very rare. The rare cases of visceral involvement such as pancreatitis have been reported in CGD (9). This case report presents a child with a previous CGD diagnosis and pleural and pericardial effusion.
2. Case Presentation
A 13-year-old boy presented with progressive dry cough from four weeks before the first admission. He was admitted to Ahvaz Hospital with left lung pneumonic involvement on a chest CT scan. He was treated with ceftazidime and vancomycin and discharged with oral cefixime. He had been treated for CGD at the age of eight, pneumonia and multiple abscesses in the brain and neck lymph nodes and mastoiditis. He had a history of brain and lymph node abscess surgery with IV and oral voriconazole. The patient had no problem for five years. Echocardiography was normal at that time. On the recent admission, the treatment was not effective. Thus, the patient was referred to our pediatric center with aggravated symptoms and retrosternal chest pain, shortness of breath, abdominal pain, dry cough, loss of appetite, vomiting, and diarrhea. He had epigastric pain worsening with eating and showed a one-kilogram weight loss in a week. The conjunctiva and skin were not icteric, and there were no skin eruptions. He had stable vital signs, no fever, and O2 saturation of 95%. The patient had no history of contact with COVID-19 patients. On initial examination, decreased heart and lung sounds in the base of both lungs were evident. Surgical wound scars were noticeable on the neck. There was no abdominal distention and no adenopathy. The patient underwent diagnostic para-clinical studies that showed PT and PTT=NL, U/A=NL, U/C=Neg., and B/C=Neg. (Table 1).
| Test | Result | Normal Range |
|---|---|---|
| White blood cell (WBC) | 10200/mm2 | 4500 - 11000 |
| Erythrocyte sedimentation rate (ESR) | 56 mm/h | 1 - 13 |
| C reactive protein (CRP) | +++ | - |
| Blood sugar | 87 mg/dL | < 140 two hours after eating |
| Blood urea nitrogen (BUN) | 19 mg/dL | 7 - 20 |
| Creatinine (Cr) | 0.5 mg/dL | 0.3 - 0.7 |
| Calcium (Ca) | 8 mg/dL | 8 - 10.3 |
| phosphorus (P) | 3.6 mg/dL | 4.8 - 7.4 |
| Aspartate transaminase (AST) | 42 IU/L | 8 - 45 |
| Alanine transaminase (ALT) | 46 IU/L | 29 - 33 |
| Lactate dehydrogenase (LDH) | 328 U/L | 140 - 280 |
| Total protein | 8.1 g/dL | 6 - 8.3 |
| Albumin | 4.1 g/dL | 3.4 - 5.4 |
| Ferritin | 570 ng/mL | 20 - 250 |
| Troponin | 0.01 ng/mL | 0 - 0.4 |
| D-dimer | 7 ng/mL | < 500 |
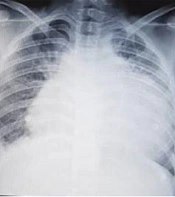
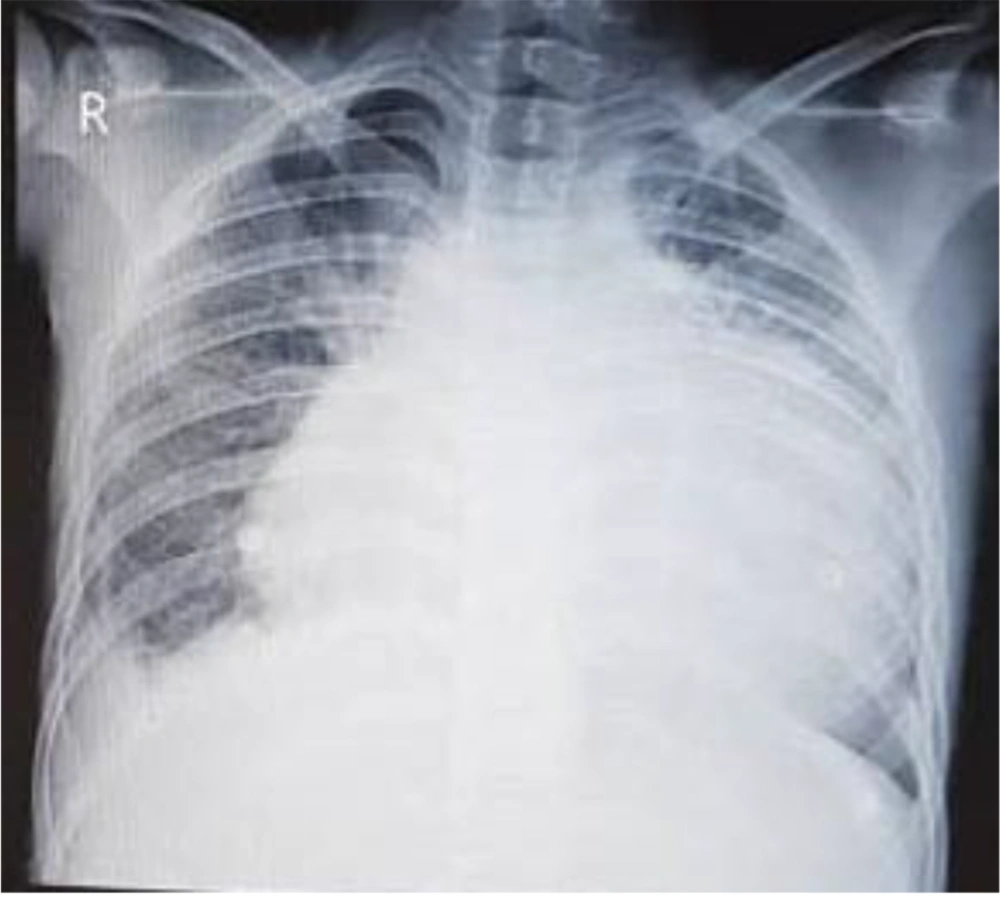
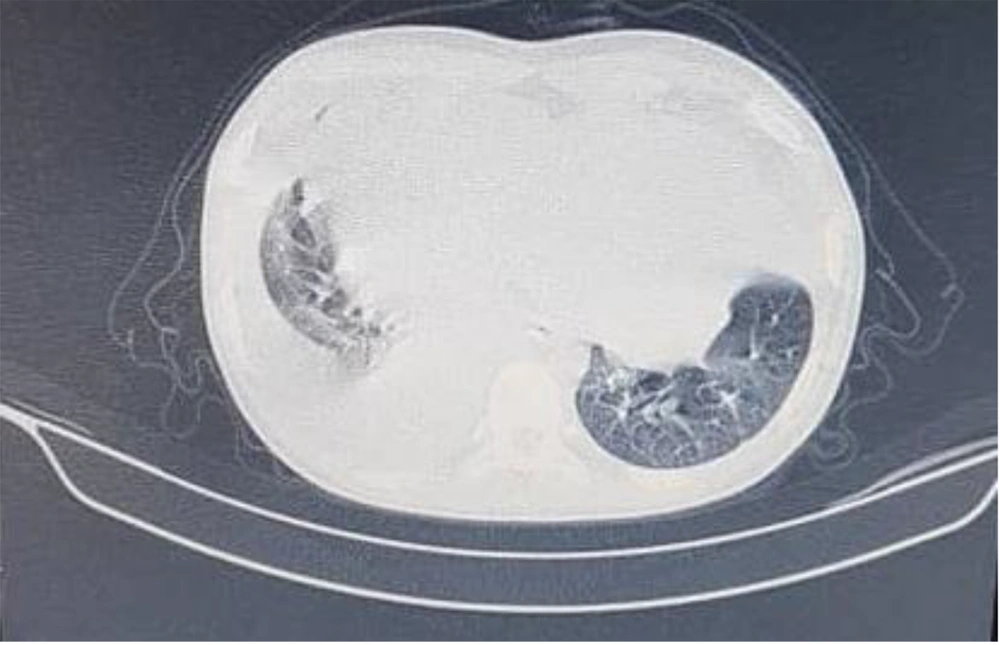
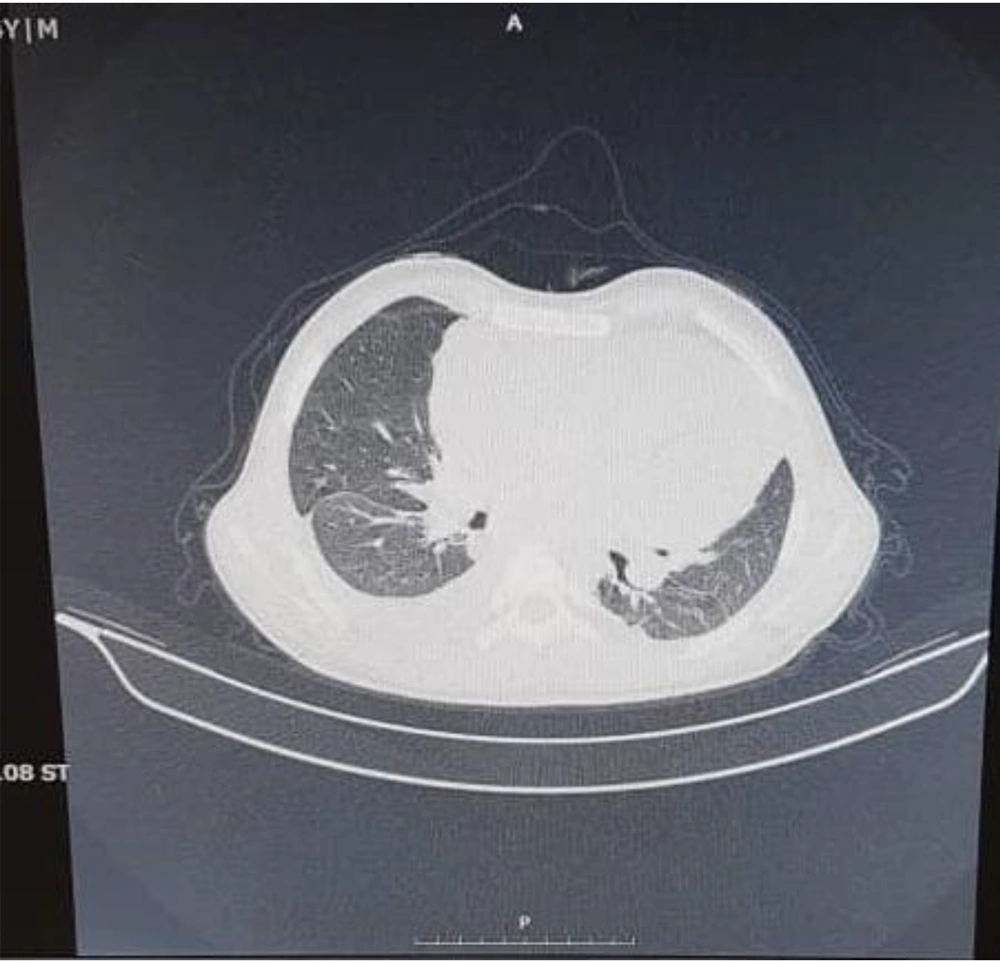
Echocardiography in our center showed massive pericardial effusion with fibrin strands and exudative pericarditis, good ejection fraction (60%), and mild TR. In chest X-ray, the heart was bottle-shaped, right CPA was blunted, and no active infiltration in the lung field was seen (Figure 1). On spiral CT scan, there was pleural effusion predominant in the right side and pericardial effusion, narrowing in the left main bronchi, and right atrial collapse related to the compression effect of pericardial effusion. Subpleural consolidation was seen in the left upper lobe anterior segment (Figures 2 and 3). A thoracentesis (Pleural tap) was performed to determine the cause of fluid accumulation. Analysis of aspirated fluid in Table 2 showed acid-fast staining for BK=Neg., pleural fluid TB PCR=Neg., fungal smear, culture, and PCR=Neg., and adenovirus, influenza, enterovirus, CMV, and EBV PCR=Neg. Also, COVID-19 and influenza nasopharyngeal PCR tests were negative. The pericardial fluid analysis had a bloody appearance, as shown in Table 3. Meropenem (30 mg/kg/dose/q 8 h/14 days), vancomycin (10 mg/kg/dose/q 6 h/14 days), and voriconazole (6 mg/kg/dose/IV/q 12 h for first 24 hours, then 4 mg/kg/dose/IV q 12 h/14 days and then orally for another 14 days) were prescribed. According to echocardiography, right atrial collapse due to large pericardial effusion was noted. Allowing effusion to drain continuously into the chest or peritoneum, the pericardial window surgical procedure was done by excision a portion of pericardium. Due to the possibility of inflammation in the pericardial space and to reduce it, steroids (methylprednisolone 1 mg/kg/dose/IV/ q 12 h started and tapered for one month orally after 14 days) were started for the patient. Pericardial biopsy specimen showed pericardial tissue with fibrosis and mixed inflammation compatible with fibrinosis, no granulomas, and no evidence of malignancy. Thereby, the patient experienced dramatic improvement. He was discharged with voriconazole, ciprofloxacin, prednisolone, naproxen, and colchicine. On a follow-up two weeks later, echocardiography was normal and physical exam showed good condition. He has been well until now. Informed consent was obtained from the patient's parents. Parents allowed their child's illness to be reported without mentioning the child's name and photo.
| Test | Result | Normal Range |
|---|---|---|
| White blood cell (WBC) | 2100/mm3 (Poly = 22% Lym = 78%) | < 300 - 1000 transudate |
| Red blood cell (RBC) | 800/mm3 | - |
| Protein | 3800 mg/dL | 1000 - 2000 |
| Glucose | 102 mg/dL | > 60 |
| Test | Result | Normal Range |
|---|---|---|
| Red blood cell (RBC) | Many/mm3 | None seen |
| White blood cell (WBC) | 1280/mm3 PMN 20%, LYMP 80% | Less than 300 |
| Lactate dehydrogenase (LDH) | 7080 U/L | less than 2/3 of the upper limit of normal serum LDH |
| Protein | 7.8 g/dL | Less than 2.5 g/dL |
| Glucose | 80 mg/dL | The same as serum value |
3. Discussion
We could not make a conclusive diagnosis for cardiac involvement based on histopathologic examination results and limitations in completing additional diagnostic tests for other differential diagnoses such as tuberculosis. This patient, however, possibly had cardiac involvement due to the underlying diseases. Since childhood, he has had infectious complications, including pneumonia, multiple abscesses, and even brain abscesses. We do not know the cause of his previous infections, but it may have been fungal disease based on his history of taking voriconazole. He had a new complication, including severe pleural and pericardial effusion, which did not respond despite prolonged antibiotic treatment. Due to a large amount of pericardial fluid, he underwent pericardiotomy, and the pericardial window was also done. Chronic granulomatous disease is a genetic defect in the function of the nicotinamide adenine dinucleotide phosphate (NADPH) complex. The defect results in the inability to produce superoxides and reactive oxygen species. This primary immunodeficiency is usually diagnosed in the first five years of life. There are five phagocyte oxidase (phox) genes in the NADPH complex (10). The most commonly involved organs are the lungs, skin, GI, lymph nodes, liver, and spleen. Pericardial involvement is rarely seen in CGD patients presenting as pericardial effusion rather than abscess formation (7). The symptoms of patients with large pericardial effusion are shortness of breath (63%), abdominal pain (63%), tachycardia (52%), and tachypnea (52%). Our case had retrosternal chest pain, abdominal pain, dry cough, and shortness of breath.
An eight-year-old boy with CGD and multiple pericardial abscesses was reported from Turkey (2016) who died despite all efforts (7). Two boys aged 10.5 months and five years with CGD and pericardial effusion were reported from England (1999). No positive culture was found in exudative pericardial fluids, and both underwent pericardial fluid extraction. The first boy received corticosteroids, and the second received antibiotic and antifungal therapy (8). One case of pericardial effusion occurred in a 20-year-old woman with CGD. The responsible organism was Aspergillus fumigates, which was treated accordingly (11). In this case, the insertion of a pericardial window combined with antibiotic and corticosteroids resulted in a complete cure, and pleural fluid subsided concomitantly. This shows the role of the inflammatory process in the pathogenesis of this problem, as the same as in some other CGD presentations (11). Pneumonic complications can be infectious or noninfectious in children with CGD. Its infectious manifestations are pneumonia, abscesses, pleural effusion, bronchiectasis, bronchitis, adenopathy, and respiratory failure, and autoimmune manifestations are granuloma formation, extensive fibrosis, pneumonitis, and Mulch pneumonitis, which is an important entity and a medical emergency treated with high-dose corticosteroids and antibacterial and antifungal drugs. Pleural effusion is rare and may occur through the inflammatory process in CGD patients (10, 12, 13). Our patient had left lung pneumonic involvement on his first admission, followed by pleural and pericardial effusion. Pulmonary manifestations such as pleural effusion can be due to autoimmune pulmonary disease (14). In our patient, antibiotic treatment for a long time could not control the process, but pleural fluid culture and other cultures were negative. The dysregulation of the inflammatory process is the main feature of CGD, which may occur as a response to a trigger and is also autoimmune in nature. It occurs as granuloma formation in multiple organs or pleural and pericardial effusion. This inflammation occurs independent of infection but incompletely resolved, or recurrent infection can cause a chronic inflammatory response, so it is difficult to exclude the presence of infection despite negative laboratory results, especially in our setting (13). In a five-year-old child with CGD and pleural effusion, the pleural fluid culture showed Pseudomonas cepacia and Pseudomonas gladioli which were successfully treated with TMP/SMX (15). Thus, in patients with CGD, pericardial and pleural effusion should be monitored closely for inflammatory and infectious causes, and management of this problem is multidisciplinary. It is also necessary to start appropriate treatment according to the pericardial and pleural effusion cause.
The limitation of this study was the lack of positive bacterial and fungal cultures in pleural fluid. This was because of laboratory restrictions in Iran, the patient's history of antibiotic use, and lack of culture and other laboratory test results in pericardial fluid, as he was under vent surgery in another hospital, and we could not obtain the results.
3.1. Conclusions
In addition to recurrent infections, CGD patients develop inflammatory and autoimmune processes. This leads to the use of steroids to reduce inflammation in addition to antibiotics in these patients. Steroid use reduces the activity of the immune system, which is impaired in these patients. Because we need long-term steroids in these patients, other drugs such as azathioprine and sulfasalazine are used for treatment. Due to high pericardial fluid and pressure on the right atrium, a pericardial window was installed in this patient. Therefore, the therapeutic challenge of these patients makes us decide on each patient separately.