1. Introduction
Healthcare-associated ventriculitis and meningitis (VM) are some of the most serious complications that drastically impact the prognosis following neurosurgical procedures. Recently, multidrug-resistant Enterobacteriaceae, especially carbapenem-resistant Klebsiella pneumonia, became an important cause of central nervous system infection, representing a major public health threat (1, 2). Such organisms represent a therapeutic contest as carbapenemase can hydrolyze all carbapenems, cephalosporins, and beta-lactams (3). The most common K. pneumoniae carbapenemase (KPC) is New Delhi metallo-β-lactamase (NDM), oxacillinase (OXA-48-like), and imipenemase (IMP) (4).
A few drugs, such as colistin, tigecycline, and aminoglycosides, and in some strains, ceftazidime/avibactam, can effectively treat CPKP. Due to the poor penetration of the blood-brain barrier, it is difficult for most medications to reach the minimal inhibitory concentration (MIC) in the cerebrospinal fluid (CSF). To overcome these disadvantages, many cases and clinical studies have attempted to treat CNSIs caused by multidrug-resistant bacteria using intraventricular (IVT) injections (4).
The best treatment for central nervous system (CNS) infection caused by carbapenem-resistant K. pneumoniae is still controversial. The current case elaborated that the combination regimen of parental, extended infusion high-dose meropenem, and amikacin could be a useful treatment option.
1.1. Ethical Consideration
Written informed consent was obtained from the patient’s parent for publishing this case report.
2. Case Presentation
A 4-month-old boy with hydrocephalus was admitted to our institute for the treatment of a suspected shunt infection that had previously been treated for three weeks with various antibiotic courses with no clinical improvement. On admission, the child was hypoactive, with a temperature of 37ºC, heart rate (HR) of 130 beats/min, respiratory rate (RR) of 30 cycles/min, and oxygen saturation of 99% in room air. His complete blood count (CBC) revealed a white blood cell count (WBC) of 6.61 × 103/cmm, absolute neutrophil count of 1.75 × 103/cmm, hemoglobin 10.5 g/dL, platelet count of 376 × 103/cm, and C-reactive protein (CRP) 9.4 mg/dL. Cerebrospinal fluid analysis (Table 1) and culture were withdrawn. The infected shunt was removed, an extra-ventricular drain was inserted, and empiric treatment with vancomycin 15 mg/kg/dose Q8h and Meropenem 40 mg/kg/dose Q8h was started. Two days later, the CSF culture revealed growth of Staphylococcus epidermidis, so meropenem was discounted, and Vancomycin was continued for 10 days.
| CSF | Day 1 | Day 9 | Day 16 | Day 23 |
|---|---|---|---|---|
| Aspect | Clear | Turbid | Turbid | Clear |
| Glucose, mg/dL | 18.59 | 30.57 | 19.24 | 40 |
| Protein, mg/dL (15.00 - 45.00) | 214.43 | 178.50 | 223.18 | 213.2 |
| LDH, U/L (0.00 - 26.00) | 86.67 | 197.30 | 151.30 | 113.7 |
| Chloride, mmol/L (110.0 - 130.0) | 115.40 | 105.20 | 104.40 | 104 |
| Neutrophil, /cmm | 120 | 44 | 25 | 0 |
| Lymphocyte, /cmm | 15 | 16 | 75 | 4 |
| Culture result | Staphylococcus epidermidis | Klebsiella pneumoniae | No growth | No growth |
On the ninth day of admission, the child became hypoactive and feverish (his temperature was 38ºC). New labs revealed an increase in CRP level 140 mg/dL, and his CBC showed the following: White blood cells 7.85 × 103/cmm, absolute neutrophil count 2.85 × 103/cmm, haemoglobin 7.7 g/dL, platelet count 157 × 103/cmm. A new CSF analysis was done (Table 1), and another portion of the CSF was sent to the molecular and diagnostic microbiology laboratory for complete identification of the pathogenic organism.
In the laboratory, 2 mL of the cerebrospinal fluid was injected into a Bact-Alert blood culture bottle (bioMérieux, France). Another portion of the CSF was cultured on Blood agar, MacConkey's agar, chocolate agar, and Sabouraud Dextrose agar (Condalab, Madrid, Spain), and a direct Gram-stained smear was examined microscopically.
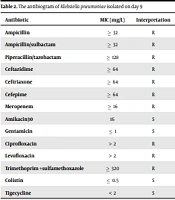
Klebsiella pneumoniae subsp. Pneumoniae was primarily identified by colony morphology and then microscopically by Gram-staining. Further identification and antibiotic susceptibility testing were done with the Vitek 2 compact system (bioMérieux, France) according to the manufacturer's protocol. The culture results showed extensive drug-resistant (XDR) carbapenem-resistant K. pneumonia (Table 2). Ceftazidime-Avibactam (CAZ-AVI) E-test (Liofilchem®, Italy), containing CAZ (0.016 - 256 µg/mL) – AVI (4 µg/mL), was used to determine CAZ-AVI sensitivity. The isolate was found to be resistant to this drug.
| Antibiotic | MIC (mg/L) | Interpretation |
|---|---|---|
| Ampicillin | ≥ 32 | R |
| Ampicillin/sulbactam | ≥ 32 | R |
| Piperacillin/tazobactam | ≥ 128 | R |
| Ceftazidime | ≥ 64 | R |
| Ceftriaxone | ≥ 64 | R |
| Cefepime | ≥ 64 | R |
| Meropenem | ≥ 16 | R |
| Amikacin30 | 16 | S |
| Gentamicin | ≤ 1 | S |
| Ciprofloxacin | > 2 | R |
| Levofloxacin | > 2 | R |
| Trimethoprim +sulfamethoxazole | ≥ 320 | R |
| Colistin | ≤ 0.5 | S |
| Tigecycline | < 2 | S |
Abbreviations: R, resistant; S, sensitive; MIC, minimum inhibitory concentration.
For genomic identification of carbapenemase genes in this bacterial isolate, bacterial genomic DNA was extracted using Thermo Scientific GeneJET Genomic DNA Purification Kit (K0721, Thermo Fisher, USA) according to manufacturer’s instructions.
SYBR green real-time PCR was performed to detect carbapenemases genes (blaNDM, blaVIM, blaIMP, blaKPC, blaGES, and blaOXA-48-like) using Mx3000PTM real-time PCR thermal cycler instrument (Stratagene, USA). A total of 6 µL of template DNA was added to the reaction mix, which was composed of 10 µL Maxima SYBR Green Master Mix (2X) (K0251 et al., USA) and 0.6 µL for each forward and reversed primers (5-7), and 2.8 µL nuclease-free water. The reaction began with an initial denaturation step at 95ºC for 10 min. This was followed by 45 cycles of DNA denaturation at 95ºC for 15 s, primer annealing at 55ºC for 30 seconds, and primer extension at 72ºC for the 30 s. This was followed by dissociation curve analysis consisting of 1 cycle at 95ºC for 1min, then at 55ºC for the 30 s, and finally at 95ºC for 30 s. The results of the molecular study showed that the bacterial isolate was positive for blaNDM, blaVIM, and blaOXA-48-like.
It was negative for blaIMP, blaKPC, and blaGES genes.
To start the effective treatment regimen, the extra-ventricular drain (EVD) was removed as biofilm formation from bacteria will hinder antibiotic penetration and lead to treatment failure. Also, inserting a new EVD was discouraged, as it may be a new source of acquiring other infections. Thus, the clinical decision was to start the treatment regimen through the parenteral route.
Following removal of the drain, we started therapy with meropenem 40 mg/kg/dose Q8h extended infusion over 4 hours accompanied with amikacin at dose 7.5 mg/kg/dose Q8hrs, and treatment was continued for 21 days. On the seventh day of treatment, a CSF culture revealed no growth, and treatment was continued for another 14 days after the negative culture.
Another two cultures were withdrawn one week apart, revealing no growth, and a new antibiotic-impregnated VP shunt was inserted after ensuring the successful treatment of bacterial meningitis.
3. Discussion
Healthcare-associated meningitis and ventriculitis are rising concurrently with an increase in neurosurgery procedures. Multidrug-resistant (MDR) K. pneumoniae CNS infection has significant morbidity and mortality (8). Meningitis caused by CRKP post-neurosurgery has been reported in the USA, Turkey, and China (9-11). The main cause of carbapenem resistance in K. pneumoniae is the production of carbapenemases, which are mainly acquired through horizontal gene transfer (12). The type of carbapenemase varies greatly in different geographical locations. The carbapenemases include KPCs, metallo-β-lactamases (MBLs), and oxacillinases (OXAs) (13, 14).
Unfortunately, which antibiotics should be used to treat CRKP infections is still unclear. Recent evidence suggests that antimicrobial combination therapy may be more effective than monotherapy and that, whenever possible, it is recommended to include meropenem as the primary component of combination regimens (15).
Parental colistin alone in treating meningitis is discouraged due to poor blood-brain barrier penetration, leading to low-dose drug concentrations in the CSF and treatment failure. Moreover, there is an increased risk of nephrotoxicity and, less commonly, neurotoxicity, even when administered in appropriate doses (16, 17). Based on the recommendation of the Infectious Disease Society of America, intraventricular treatment is necessary for healthcare-associated ventriculitis and meningitis where systemic antibiotic therapy is ineffective (18).
Given the data mentioned above, and though meropenem resistance (MIC ≥ 16 mg/L) to the isolated CRKP strain, in this case, the selected treatment regimen with high dose extended infusion of meropenem and amikacin was administered, and the patient was effectively cured.
The rationale for using this regimen depends on the fact that carbapenems have time-dependent antibacterial activity; the antibacterial activity is correlated with the length of time that free concentrations persist above the minimum inhibitory concentration (MIC) (time of free concentration (%fT) > MIC). For carbapenems to be effective against bacteria in severe infections, larger levels (%fT > MIC, 70 to 80%), or even higher), are required. As a result, adopting the maximal dose typically necessitates extending the infusion period (3 to 4 h or continuous infusion) for improved pharmacokinetic (PK) and pharmacodynamic (PD) outcomes (19).
In concordance with our case, He et al. also reported successful treatment of carbapenem-resistant Enterobacter cloacae (MIC of imipenem ≥ 16 mg/L) with high dose and prolonged-infusion regimen of IV meropenem and Amikacin plus Intraventricular Amikacin (20). Different treatment regimens were used in different other studies, including parental tigecycline and amikacin with intraventricular amikacin, meropenem and intraventricular colistin, and IV ceftazidime/avibactam and intraventricular gentamicin (9-11).
3.1. Conclusions
This case report demonstrates the possibility of using a high dose of extended infusion meropenem and Amikacin in treating CRKP Meningitis (MIC ≥ 16 mg/L) precisely with limited treatment options. Further studies are urgently needed to determine the most effective treatment of CRKP meningitis. This case report sheds light and paved the way in treating CRKP Meningitis and proves treating CRKP Meningitis and proves that antimicrobial combination therapy will be more effective than monotherapy.
It is recommended to include meropenem as the primary component of combination regimens.