1. Background
Diarrhea, as a major health problem, is considered a leading cause of morbidity and mortality, especially among children, in developing countries (1). Salmonella and shigella species are two major enteropathogenic bacteria, which, along with Enterotoxigenic Escherichia coli (ETEC) and Campylobacter, account for the most diarrheal infections in the developing world. Human salmonellosis, being more prevalent among children under 5 years of age, causes more than one billion disease cases annually, 3 million of which lead to death across the world (2, 3).
In addition, about 200 million Shigella infections and 3 - 5 million deaths occur annually in developing countries, most of which affect children under 5 years of age (2). Epidemiological data have shown that Shigella sonnei (S. sonnei) is the predominating Shigella species in Europe and USA while Shigella flexneri (S. flexneri) is more frequent among Asian and African countries (4). In Tehran, until 2003, the dominant species was S. flexneri, but it was later replaced by S. sonnei (5).
Salmonella and particularly Shigella species are increasingly acquiring resistance to commonly used antibiotics, most importantly to third-generation cephalosporins (3GC), as the main drug of choice for the treatment of Salmonella and Shigella infections, through the production of extended-spectrum β-lactamases (ESBLs) (6-9). Data on local antibiotic resistance patterns are essential to have an empiric antibiotic treatment guideline adapted to the local microbial epidemiology (10).
2. Objectives
The aim of the present study was to determine the prevalence and drug susceptibility patterns of Shigella and Salmonella isolates in addition to the detection of ESBL producing isolates among diarrhea samples of pediatric patients.
3. Methods
3.1. Sample Collection
A cross-sectional study was conducted from 2012 to 2016 on pediatric patients admitted to the children’s medical center (CMC) hospital in Tehran, Iran, to assess the prevalence and drug susceptibility patterns of Salmonella and Shigella species, in addition to the detection of ESBL producing isolates. Single stool samples from each of the 5,300 diarrheic children were examined for the presence of Salmonella and Shigella species. All the patients, admitted with the clinical signs of acute diarrhea or enteric fever, had an Iranian nationality with an age range of 1 - 10 years old.
3.2. Bacterial Analysis
Stool specimens were cultured on MacConkey (Merck Co., Germany) and Xylose-Lysin-Deoxycholate (XLD) selective media (Merck Co., Germany) and incubated at 35 - 37° C for 1 to 4 days. Inoculation into Selenite-F enrichment broth (Oxoid Co., UK) and subculture on XLD after 8 - 12 hours were then performed to improve the recovery of Salmonella spp.
All the suspected colonies grown on XLD were analyzed by routine biochemical and microbiological tests (11). Specific polyvalent antisera (Baharafshan Co., Iran) were used for the serotype identification of Salmonella and Shigella species.
3.3. Antimicrobial Susceptibility Testing and Detection of ESBLs
Antibiotic susceptibility tests were performed by the Kirby-Bauer disc diffusion method on Mueller Hinton agar (Merck Co., Germany), as recommended by the clinical and laboratory standards institute (CLSI) (12). The antimicrobial agents (MAST Co., UK) used in this study were ampicillin (10 µg), nalidixic acid (30 µg), co-trimoxazole (25 µg), and cefotaxime (30 µg). E. coli ATCC (American type culture collection) 25922 was used as the control.
The phenotypic detection of ESBL production was carried out for Salmonella and Shigella spp. that were resistant to cefotaxime and ceftazidime by the combination disc method using the cefotaxime (30 µg), cefpodoxime (30 µg), and ceftazidime (30 µg) discs (MAST Co., UK), with or without 10 µg of clavulanic acid. A ≥ 5 mm increase in the zone diameter of ceftazidime, cefpodoxime, or cefotaxime tested in combination with clavulanic acid versus its zone tested without clavulanic acid was indicative of ESBL production. The ESBL-producing strain K. pneumoniae ATCC 700603 was used as the positive control (12).
3.4. Statistical Analysis
The Chi-Square test was used to analyze the correlation between the prevalence of Salmonella and Shigella species and the age and gender of the patients, as well as the resistance rate of Salmonella and Shigella species with respect to each antibiotic.
SPSS version 22 was used for the analyses and P value ≤ 0.05 was considered significant.
4. Results
4.1. Bacterial Isolation
Out of the 5300 stool samples collected, 371 (7%) and 472 (8.9%) were positive for Salmonella and Shigella species, respectively. Of the 371 Salmonella isolates, 64 (17.2%) belonged to group B, 131 (35.3%) to group C, and 176 (47.5%) to group D.
Of the 472 Shigella isolates, 287 (60.8%) were identified as S. sonnei and the remaining 185 (39.2%) as S. flexneri. In this study, the prevalence of S. flexneri and S. sonnei was significantly higher among patients older than 5 years of age (P < 0.001). All the Salmonella serogroups were significantly higher in prevalence among patients in the age group of 1 - 5 years (P < 0.001).
The distribution of Salmonella and Shigella species according to the sex and age of the patients and their distribution among different hospital’s wards are respectively shown in Tables 1 and 2.
| S. flexneri | S. sonnei | S. Serogroup B | S. Serogroup C | S. Serogroup D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Age group, y | ||||||||||
| < 1 | 2 (40) | 3 (60) | 4 (80) | 1 (20) | 9 (50) | 9 (50) | 27 (64.3) | 15 (35.7) | 28 (56) | 22 (44) |
| 1 - 5 | 46 (52.3) | 42 (47.7) | 69 (53.1) | 61 (46.9) | 19 (50) | 19 (50) | 34 (53.1) | 30 (46.9) | 52 (50) | 52 (50) |
| 5 - 10 | 52 (56.5) | 40 (43.5) | 87 (57.2) | 65 (42.8) | 5 (62.5) | 3 (37.5) | 16 (64) | 9 (36) | 12 (54.5) | 10 (45.5) |
| Total | 185 | 287 | 64 | 131 | 176 | |||||
Abbreviations: S. flexneri, Shigella flexneri; S. sonnei, Shigella sonnei; S. serogroup B, Salmonella serogroup B; S. serogroup C, Salmonella serogroup C; S. serogroup D, Salmonella serogroup D.
aValues are expressed as No. (%).
| Organisms | Hospital Wards | ||||||
|---|---|---|---|---|---|---|---|
| Outpatients | Emergency | Infectious Diseases | Gastroenterology | Rheumatology | ICU | Total | |
| S. sonnei | 177 (61.7) | 100 (34.8) | 5 (1.7) | 2 (0.7) | 2 (0.7) | 1 (0.3) | 287 |
| S. flexneri | 99 (53.5) | 60 (32.4) | 19 (10.3) | 6 (3.3) | - | 1 (0.5) | 185 |
| S. serogroup B | 44 (68.7) | 12 (18.7) | 3 (4.7) | 3 (4.7) | 1 (1.6) | 1 (1.6) | 64 |
| S. serogroup C | 78 (59.5) | 25 (19.1) | 9 (6.9) | 3 (2.3) | 14 (10.7) | 2 (1.5) | 131 |
| S. serogroup D | 106 (60.2) | 45 (25.6) | 8 (4.6) | 11 (6.2) | 3 (1.7) | 3 (1.7) | 176 |
Abbreviations: S. flexneri, Shigella flexneri; S. serogroup B, Salmonella serogroup B;S. serogroup C, Salmonella serogroup C; S. serogroup D, Salmonella serogroup D; S. sonnei, Shigella sonnei; ICU, intensive care unit.
aValues are expressed as No. (%).
4.2. Antibiotic Resistance Patterns
With 96.2% resistance to ampicillin, 85.4% to co-trimoxazole, 39.5% to cefotaxime, and 37.3% to nalidixic acid, S. flexneri was the most resistant isolate among all the Shigella isolates tested.
Among the Salmonella serogroups, group C had the highest level of antibiotic resistance (68.7% resistance to nalidixic acid and 48.8% to co-trimoxazole). Resistance to ampicillin and co-trimoxazole was significantly higher among Shigella spp. than among different Salmonella serogroups (P < 0.001).
On the other hand, Salmonella serogroups had significantly higher resistance to nalidixic acid compared to Shigella species (P < 0.001).
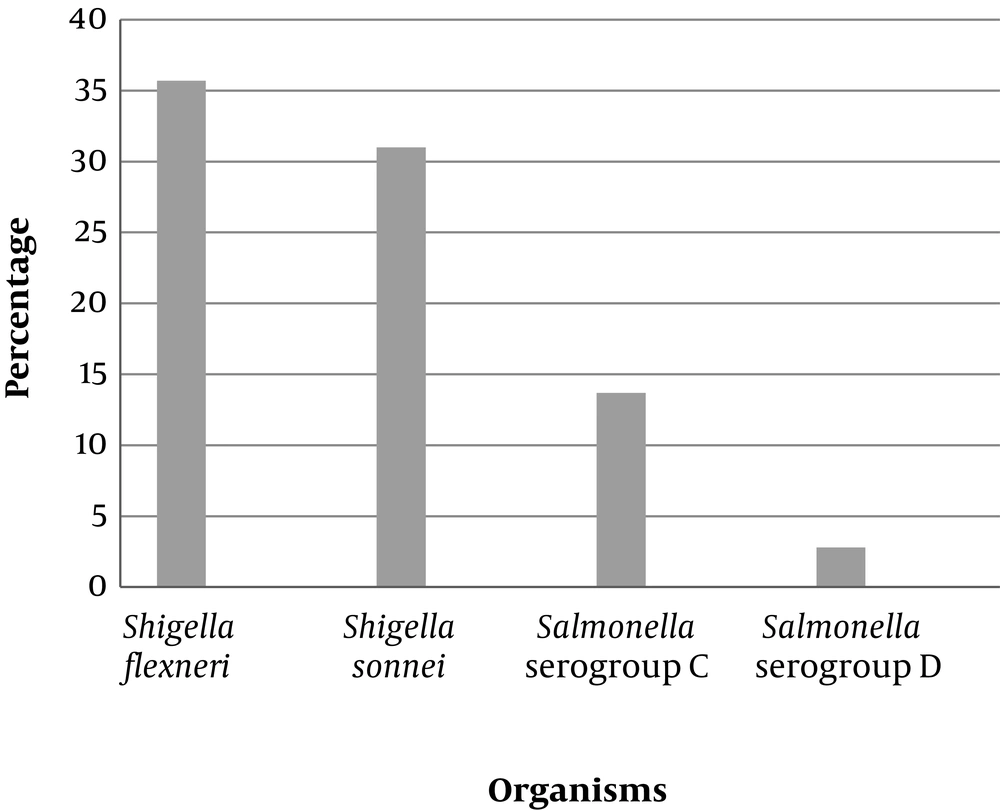
The results of the antibiotic resistance patterns and ESBL detection tests for the Salmonella and Shigella isolates are presented in Table 3 and Figure 1.
| Organisms | Antibiotics | |||
|---|---|---|---|---|
| Ampicillin (10 µg) | Co-trimoxazole (25 µg) | Cefotaxime (30 µg) | Nalidixic Acid (30 µg) | |
| S. sonnei | 105 (36.6) | 280 (97.6) | 96 (33.4) | 165 (57.5) |
| S. flexneri | 178 (96.2) | 158 (85.4) | 73 (39.5) | 69 (37.3) |
| S. serogroup B | 6 (9.4) | 11 (17.2) | 0 (0) | 11 (17.2) |
| S. serogroup C | 23 (17.6) | 64 (48.8) | 19 (14.5) | 90 (68.7) |
| S. serogroup D | 16 (9.1) | 9 (5.1) | 6 (3.4) | 129 (73.3) |
Abbreviations: S. flexneri, Shigella flexneri;S. serogroup B, Salmonella serogroup B; S. serogroup C, Salmonella serogroup C; S. serogroup D, Salmonella serogroup D; S. sonnei, Shigella sonnei.
aValues are expressed as No. (%).
5. Discussion
Salmonella and Shigella infections are global public health concerns that are increasingly acquiring resistance to commonly used antibiotics, posing serious problems in antimicrobial treatment worldwide.
In this study, the prevalence of Salmonella spp. was 7%, which is similar to that reported by Awole et al. from Ethiopia (7.2%) (13) and lower than those found in other studies as 11.5% (14) and 15.4% (15).
The prevalence of Shigella spp., in the current investigation, was 8.9%, which is similar to that reported by studies from Ethiopia (8.7%) (16) and South Africa (8.5%) (17), but higher than those reported in other similar studies in Iran as (5.1%) and (3.8%) (18, 19). The prevalence of shigellosis reported in other developing countries such as Cameroon (4.5%) (20), India (5%) (21), and Ghana (5%) (22) was also lower than that observed in the current study.
This discrepancy may be due to the levels of public health and the availability of safe drinking water among populations, the source of samples, sample size, geographical distribution, the season in which samples were collected, differences in personal hygiene and the age of the target group.
Based on the results of the present study, low levels of Salmonella resistance was observed to ampicillin (12%), which is lower than that reported by Jimma (59.3%) (15) and Harar (71.4%) (14). Salmonella isolates were highly susceptible to cefotaxime (94.1%), which was similar to that found by Hamze et al. (23) but dissimilar to the findings of Bialvaei et al. (9).
Our results showed that among the Salmonella serogroups, group C had the highest level of antibiotic resistance to nalidixic acid (68.7%) and co-trimoxazole (48.8%). Various studies have reported different levels of resistance to these antibiotics; for instance, Anvarinejad et al. found 31.5% and 15.79% resistance to nalidixic acid and co-trimoxazole, respectively (3). In another study carried out by Mamuye et al., resistance to nalidixic acid and co-trimoxazole was reported, respectively, as 20% and 60% (8). Moreover, 93.4% of Salmonella spp. was resistant to co-trimoxazole as reported by Bialvaei et al. (9).
In this study, S. sonnei (60.8%) was the most common Shigella species followed by S. flexneri (39.2%), which is in contrast with the results of studies by Jomezadeh et al. (18) and MoezArdalan et al. (24) that showed S. flexneri as the most prevalent Shigella species in Abadan and Karaj, respectively. Our results, however, are in agreement with the results of studies from Shiraz and Tehran (25, 26), confirming recent reports claiming a change over the last years in the prevalence of Shigella spp. in Tehran (5).
In this study, most of the Shigella isolates were resistant to ampicillin and co-trimoxa-zole, which is in accordance with the previous studies from Iran (18, 27) and other countries such as Nepal (28) and India (29). The wide use of co-trimoxazole and ampicillin, as broad spectrum and low priced empirical treatments for diarrheal diseases (30), has led to the advent of resistance of Shigella strains to these antibiotics. According to the results of the present study, these two antibiotics are no longer suitable choices for the treatment of Shigella infections. It seems that resistance to nalidixic acid, as the first line of treatment for diarrheal infections, is on the rise in Iran. A study carried out in 200 - 2003 by MoezArdalan in Iran showed that only 1% of the Shigella isolates were resistant to nalidixic acid (24), whilst our study showed a rise of 47.4% resistance to this antibiotic. In other countries, like India, the resistance of S. sonnei to nalidixic acid was reported as 94% - 100% (31, 32).
In this study, 39.5% of S. flexneri and 33.4% of S. sonnei isolates were resistant to cefotaxime, which is consistent with the results of Bialvaei et al. (9) but much higher than the prevalence found by Barari Savadkoohi et al. (33) and Mamuye et al. (8). Moreover, 35.7% of S. flexneri and 31% of S. sonnei isolates produced ESBL in the present study, which is in agreement with the results of Bialvaei et al. (9) but higher than those reported by Nath et al. (2.8%) (21), Ranjbar et al. (7.3%) (7), and Sakhaei et al. (11.4%) (34).
ESBL producing Shigella species can be problematic in near future and therefore, treatment guidelines should be designed to monitor the emerging ESBL producing isolates at a national level (21).
High rates of antibiotic resistance may result from the uncontrolled or inappropriate use of antibiotics. This can cause antibiotic-resistant bacteria to spread easily between hospital wards, leading to protracted outbreaks with high mortality and morbidity rates (35). The epidemiological information and monitoring system are, therefore, necessary to control Salmonella and Shigella infections in public health sectors.
5.1. Conclusion
This study provided information on the prevalence and antimicrobial susceptibility patterns of Salmonella and Shigella infections in Tehran, Iran. The antimicrobial resistance patterns suggest the widespread resistance of Shigella to ampicillin and co-trimoxazole, which requires specific attention because co-trimoxazole is considered the drug of choice for the treatment of patients with inflammatory diarrhea. Due to the continuous change in antibiotic resistance profiles, Salmonella and Shigella strains should be under-scrutinized surveillance in order to monitor local susceptibility patterns for the administration of appropriate antibiotics.