1. Context
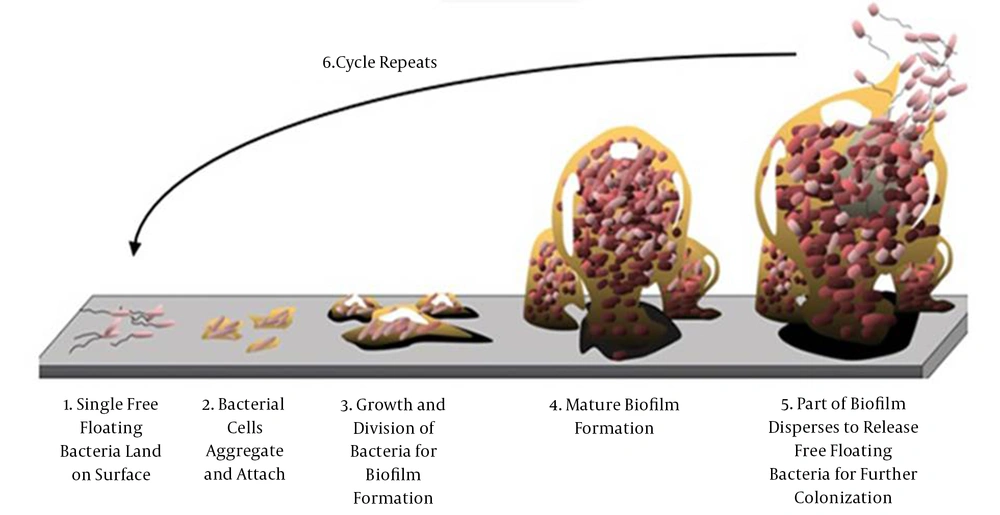
Biofilms mainly comprise microbial cells and exopolysaccharides (EPS) (1) and connect to abiotic surfaces. The adhesion step is essential for the bacteria arranged in their environmentally favorable conditions (2). Bacterial growth is characterized by planktonic or sessile aggregates. Sessile aggregates are commonly referred to as a biofilm and contain multiple bacteria forming a consortium (3). Figure 1 shows the stages involved in the formation and life cycle of a biofilm. A biofilm acts as a protection factor for the bacteria against antimicrobial and host immune system attacks and favors infection chronicity. Quorum sensing (QS) systems is a cellular recognition mechanism which regulate biofilm formation. Since biofilm formation can pose remarkable problems in the Health settingby, stimulating further resistance to the treatment with antibiotics and biocides, it even decreases host immune responses (4).
Adhesion step and sessile aggregation of a biofilm (5)
The pathophysiology of biofilms is involved in the bacteria colonization process and disseminative stages. In the disseminative stages and colonization processes, the bacteria emerge in a planktonic form; however, the biofilm formation steps are determined by cellular accumulations in a distinguished form and physiology (6).
Microbial biofilms are normally out of access to antibiotics and the human immune system. Biofilm-producing bacteria present resistant phenotypes due to some changes e.g., nutritional status or cell density, temperature, osmolarity, and pH) (7).
According to the statistics, about 80% of clinical infections in human are associated with biofilms. Due to the effect of microbial biofilms on the care and treatment strategies, millions of dollars have been spent in ambiguity for a long term. A biofilm is quickly produced on stainless steel and titanium orthopedic bolts and encompasses powerful adhesion forces to stick to foreign bodies by some bacteria such as Staphylococcus aureus, S. epidermidis, and Pseudomonas aeruginosa (8, 9). Some diseases induced by biofilm formation are pneumonia, skin infections, food poisoning, endocarditis, osteomyelitis, soft tissue infections, septic arthritis, cystic fibrosis, periodontitis, and UTI, which are mainly caused by indwell medical devices and life-threatening invasions (10-19). In some microorganisms, cell-cell signaling during sporulation exhibits different cellular forms containing distinguished cell types, thereby enhancing survival via the labor division (20). There are some medically-significant fungi producing biofilms such as Candida, Pneumocystis, Aspergillus, Trichosporon, Coccidioides, and Cryptococcus (21). Unfortunately, recent reports have described a worldwide increase in the prevalence of these organisms as well as high mortality and morbidity rates in healthcare settings as such, they have been a major concern worldwide. To make appropriate policies, national information is required to revise the essential drugs’ list for treatment and evaluate the effects of intervention strategies (22). Biofilm formation as a virulence determinant may result in the development of multi-drug resistance. For example, Acinetobacter is the leading cause of hospital-acquired infections worldwide. In this regard, there are some reasons as follows: (1) tendency to create someinfections in critically-ill patients; (2) predilection to develop resistance to multiple antibiotics; and (3) survivability for a long term on equipment and hospital surfaces, and several outbreaks attributed to common source contamination. Moreover, the MDR, XDR, and PDR (representing multi-, extensive- and pan-drug resistant) strains of A. baumannii have been reported worldwide (23-25).
There is an urgent need to study factors in charge of spreading antibiotic resistance and gene transfer. Some genes associated with biofilm formation result in treatment failure among wound infections due to the involvement of some highly resistant bacteria (26). Wound infection in burned patients should be demonstrated as a potential risk due to antibiotic-resistant bacteria such as P. aeruginosa, Acinetobacter, and Klebsiella (4).
Moreover, in burn centers, the rapid acquisition of MDR result in high morbidity and mortality rates. After exposure to various antibiotics and cross-resistance may emerge some resistant strains such as MDR, XDR, and PDR. Consequently, given the rapid emergence of hospital antibiotic-resistant pathogens, the periodic assessment of antibiogram sensitivity and bacterial colonization patterns in burn wards seems crucial (27).
During the last decade, the significance of biofilms in clinical settings was documented. Infections induced by biofilms are observed in all parts of human body. Now, biofilm-forming bacteria seem to be associated with chronic infections (28).
Accordingly, this study described an updated review of biofilm matrix formation in human regarding its clinical significance, diagnosis techniques, and therapeutic drugs.
2. Evidence Acquisition
In this narrative review, a comprehensive review of the relevant literature further revealed structural and functional variations. Moreover, it was noticed that biofilms play a role in disease and host-pathogen interactions. The present study describes the most recent information on the significance of microbial biofilm formation in clinical settings, common biofilm producing bacteria, antibiotic drug resistance, its diagnostic methods, and new approaches to the treatment of infection induced by biofilm formation.
3. Results
3.1. Significance of Biofilms in Clinical Settings
According to a comprehensive review of the literature, about 99.9% of bacteria produce biofilms at biological and inanimate levels. Biofilm producing has been reported in a large number of Gram-positive (S. epidermidis, S. aureus, Actinomyces israelii) and Gram-negative (E. coli, Klebsiella pneumonia, Enterobacter cloacae, Pseudomonas aeruginosa, Burkholderia cepacia, Haemophilus influenza, and A. baumannii) bacteria (29-32).
Some infections are associated with residing prosthesis and implantable devices. Tables 1 and 2 present the list of human infections induced by indwelling devices and those involving biofilms (33, 34). Table 3 also presents human infections induced by indwelling devices, and Table 4 shows human infections caused by biofilm-producing bacteria. A list of some medical devices by which biofilms can develop is presented in Table 5. Some medical devices, by which biofilms can develop, are as follows: (1) prosthetic joint; (2) endotracheal tubes; (3) tympanostomy tubes; (4) peritoneal dialysis catheters; (5) voice prostheses; (6) pacemakers; (7) urinary catheters; (8) contact lenses; (9) mechanical heart valves; (10) central venous catheters; (11) intrauterine devices; and (12) central venous catheter needless connectors (35).
| Variables | Values |
|---|---|
| General aspect of clinical signs | (In some cases) low-grade fever, loss of function, dolor, low-grade inflammatory reactions tumor, and rubor; Implanted medical device and cystic fibrosis (CF) disease as the medical history of biofilm-predisposing situations; All persistent and lasting (> 7 days) infections, antibiotic resistance during treatment; Infection relapse and antibiotic treatment defeat; Documented record /antibiotic defeat background; All systemic signs and infection symptoms resolve with antibiotic therapy. |
| Microbiological diagnostics | A- Microscopic evidence of tissue/ fluid samples gathered from suspected infection location; Microscopic evidence disclosing microbial aggregates by the examination of fluid sample or smear; Microscopic evidence Microscopic evidence disclose microbial co-localized together with inflammatory cells; Microbiological document confirm with infectious etiology |
| B- Microbial microorganisms indicated by a few procedures (e.g., positive culture/non-culture-based (PCR) of tissue or liquid example) | Microbial pathogens detected by culture (MALDI-TOF); Mucoid settlements or variations of Pseudomonas aeruginosa small colony in culture-positive examples, indicating anti-infection disobedience; Positive outcomes detected by a few molecular techniques (e.g., PCR, quantitative PCR, or multiplex PCR for microbes related to contamination with P. aeruginosa and CF, Staphylococcus aureus related to contamination with implant); The positive outcome of fluorescence in situ hybridization for well-known pathogens displaying snuggled microorganisms; Microbial pathogen detected by several non-culture-based techniques such as next-generation sequencing, pyrosequencing, specific immune reaction to known bacteria such as P. aeruginosa antigens in patients with CF (if infections induced by biofilm last for about 2 weeks.) |
| Anatomic site A with normal flora | Anatomic site B without normal flora |
|---|---|
| Skin; Pharynx; Duodenum; Urethra; Vagina; Air in operating room, skin flora | Blood, peritoneum; Bronchi, lungs; Bile tract, pancreas; Bladder; Uterus; Neurosurgical shunt, alloplastic material |
| No symptoms | Pathology |
| Device | Infection |
|---|---|
| Peritoneal dialysis catheters | Exit-sit-infections, peritonitis |
| Hemodialysis catheters | Access site infections, endocarditis, bacteremia |
| Urinary catheters | Urinary tract infections, bacteremia |
| Intravenous catheters | Access site infections, bacteremia |
| Prosthetic cardiac valves | Prosthetic valve endocarditis, bacteremia |
| CSF shunts and reservoirs | Access site infection, meningitis |
| Cardiac pacemakers | Lead and generator infections, endocarditis |
| Contact lenses | Conjunctivitis, endophthalmitis |
| Surgical sutures & staples | Urinary tract infections |
| Infection | Common Biofilm-Producing Bacteria |
|---|---|
| Musculoskeletal infections, acidogenic enzymes of dental caries plaque | Gram-positive cocci (e.g., Streptococcus) |
| Serious gum infection (periodontitis) | Oral anaerobic bacilli (Gram-negative) |
| Inflammatory diseases of middle ear or otitis media (OM) | Haemophilus influenzae (nontypable strains) |
| Necrotizing fasciitis (NF) | Group A streptococci |
| ICU pneumonia, biliary tract infection, urinary catheter cystitis, bacterial prostatitis | Enterobacteriaceae |
| Endocarditis of native valve | VGS (viridans group streptococci) |
| Cystic fibrosis (CF), meloidosis | Pseudomonadaceae |
| Scleral buckles, vascular grafts | Cocci (especially Gram-positive) |
| Ocular prosthetic devices, such as contact lens | Cocci (especially Gram-positive), and Pseudomonas aeruginosa |
| Continuous ambulatory peritoneal dialysis, osteomyelitis, endotracheal tubes, peritonitis | Wide assortment of microbes, fungi |
| IUDs | Actinomyces israelii and wide assortment of microbes |
| Hickman line, central venous catheters (CVC) | Staphylococcus epidermidis, Candida albicans, and others |
| Bile duct stent | Enterobacteriaceae, fungi |
| Method | Application | Objectives |
|---|---|---|
| Roll plate | Identification of extra-luminal biofilm | Bacterial growth related to biofilm |
| Plate counting, Sonication, vortex and | Identification of intra-luminal, and extra-luminal biofilm | Bacterial growth related to biofilm |
| Staining by acridine orange | Identification of extra-luminal biofilm | Direct investigation microscopy of biofilm on catheter |
| Streak plating | Biofilm examination delivered on an inhabiting catheter | Bacterial growth related to biofilm |
Biofilms associated with central venous catheters (CVC) are used to provide fluid, blood products, nutrition or medications, and access to the dialysis treatment (7, 35). However, the contamination of the external or internal parts of the catheter leads to biofilm formation. After the first week of catheterization, extra luminal biofilm can cause catheter-associated bloodstream infections. Conversely, some evidence from luminal colonization and biofilm formation is obtained after indwelling vascular catheters for more than 30 days. Accordingly, patients requiring the long-term use of devices (e.g., patients with bone marrow transplantation) are at greater risk of developing blood infections (37, 38).
Biofilms can stick to the material of mechanical heart valves and heart surrounding tissues, inducing a disease called prosthetic endocarditis. In this regard, some of the main bacteria associated with this disease are Gram-negative bacilli, diphtheroids, Candida spp., S. epidermidis, S. aureus, Streptococcus spp., and enterococcus spp. These organisms may appear because of using devices such as CVC or following dental procedures (7).
The nature of causative agents is associated with their origin. Some contamination-arousing organisms (e.g., early endocarditis induced by S. epidermidis) originate at the surgery time. Some organisms (e.g., Streptococcus spp.) emanate after dental work. Because of indwelling a medical device, several organisms may exhibit infections. After residing the mechanical heart valve, there may be some events such as the accumulation of circulating platelets, fibrin attachment to the valve, and tissue damage. Similarly, the bacteria’s greater tendency to attach to these locations is documented. Moreover, biofilms reach the tissue around the prosthesis or can stick to the synthetic medical devices. Generally, antimicrobial drugs are administered after the valve replacement and dental procedures to prevent early sticking by killing all bacteria spread into the bloodstream. According to some studies, only a small number of patients can be released from an infection induced by biofilm only by adopting antibiotic treatment (31, 32, 37).
Catheters are commonly necessary for patients not capable of voiding. A urinary catheter is a hollow, construction rubber or plastic (PVC) silicone, flexible tube in several sizes and types, which gathers urine from the bladder and propels it to a drainage bag. The disruption in emptying the bladder can pose pressure on kidneys. Kidney dysfunction occurs following such pressure, which can be hazardous and causes kidneys’ persistent damage. Catheters are often used for a short period of time; however, the elderly and patients with a persistent injury or drastic illness may need catheters for a long time (38). A catheter-induced infection was increased by about 10% per day of using the catheter. Both the internal and external surfaces of urinary catheters can be readily expanded by biofilms colonization, and this condition cannot be deviated merely via hygiene scale. Hence, clinicians should prescribe catheters only in the case of necessity and forbear catheterization for a prolonged or continuous period (7). The contamination may be aroused by those bacteria colonized in the periurethral region. They can move into the bladder via the mucosal lining that is within the epithelial cells or the urethra region and the catheter. Urine contamination may happen in the catheter drainage bag and induce infections in that patient. IN this regard, the most frequent strategy is the catheter elimination and substitution. Catheter disruption and displacement can pose further complications; hence, biofilm can sheds planktonic cells or aggregates of cells after indwelling device and provides bacterial spread to other anatomical places in body. It is proved that urease production by some bacteria increases the urinary pH and promotes crystalline biofilm formation in the urinary catheter. Sometimes, crystalline biofilms appear on the catheter’s external surface (e.g., catheter tip and surrounding balloon), causing trauma to the urethral epithelia and bladder. Then, because of stone formation, biofilm debris can cause obstruction in the bladder. Furthermore, the crystalline biofilm can cause catheter lumen obstruction and urine flow obstruction by the catheter (33).
Recurrent UTIs are popular among young, healthy women, even those whose urinary tracts have a normal structure anatomically and physiologically. One of the substantial burdens to the healthcare system is that almost 25% of women with acute cystitis experience recurrent UTIs. In this regard, some studies have highlighted the significance of detecting the main factors causing recurrent UTIs to expand effective prevention methods and therapies (39). Relapse induced by uropathogenic E. coli (UPEC) is associated with these strains’ potentials for biofilm formation. As a result, biofilm formation is the main feature of the UPEC persistence not only in the vaginal reservoir but also in the bladder epithelial cells (39-44).
3.2. Antibiotic Drug Resistance
Compared to planktonic cells, biofilm-producing bacteria adopting several mechanisms can be more resistant against antibiotics as much as 1000 times (45-48). Some of the mechanisms are as follow: (1) antibiotic diffusion is limited via the matrix: for example, aminoglycosides penetrate via the matrix more slowly compard to β-lactams drugs; (2) resistance genes transmit across the community: Mobile genetic elements such as plasmids, transmits between cells by close contact, leading to spreading resistance markers; (3) the expression of efflux pumps is a drug resistance mechanism both in planktonic cells and in biofilm-producing bacteria; (4) antibiotics are inactivated by some changes in pH and metal ion concentrations. Each pH change in biofilms can inactivate antibiotics by the antibiotic activity anatomization; (5) metabolically, inactive cells denominate the persister cells. Persister cells are regular and dormant variants, which are not mutants. They act as a surviving cell reservoir for rebuilding the biofilm population (49). The acquisition of such a persisted status is mediated by toxin-antitoxin modules (50). The resistance level is associated with the biofilm formation stage. When bacteria have not connected themselves in the matrix, they become vulnerable against the actions of host immune system and antibiotics (51). In general, biofilm formation is accompanied by further resistance against host immune responses and antibiotics (52). The cells’ characteristics in the matrix are protected from being exhibited to the innate immune system in antimicrobial therapy; hence, the communication of biochemical signals between them is facilitated (53). Moreover, it was demonstrated that the bacteria resistance in biofilm against the large number of antibiotics can be related to culture physiological conditions and density, not to their existence in the biofilm. On the other hand, the distribution of virulence factors and resistance characters can be enhanced by their construction (54). It was proved that biofilm formation raised the potentials of S. aureus against antibiotic resistance caused by plasmid by means of both mobile genetic elements, namely mobilization, and conjugation. This could be facilitated by close cell-to-cell contact in biofilms and may be prospered by the biofilm matrix (55).
3.3. Diagnosis: Assays and Lab Devices of Biofilm Formation Evaluation
In laboratories, microdilution and broth macrodilution methods are routinely used for detecting the antimicrobial activity of agents against planktonic microorganisms. The methods are introduced by National Committee for Clinical Laboratory Standards (NCCLS), European Committee on Antimicrobial Susceptibility Testing (EUCAST), and Clinical Laboratory Standards Institute (CLSI). They, however, have never yield accurate results in biofilm producer microorganisms (2). There are several methods to detect microbial biofilm in response to agents (Tables 6 - 8). Moreover, several devices, including disk reactor, perfused biofilm fermenter, model bladder, Centers for Disease Control (CDC) biofilm reactor, Calgary biofilm device, and modified Robbins device, have been improved as the model system. Providing information about biofilm mechanisms, these model systems define antimicrobial drugs susceptibility against biofilm-producing bacteria (36).
| Method | Aim |
|---|---|
| TM | Tube method: A qualitative method monitoring biofilm formation lined on tube walls and bottom. |
| CRA | Congo red agar: A qualitative method monitoring the color change of colony. |
| MtP | Microtiter plate: A quantitative method using micro ELISA or microplate reader. |
| PCR (including real-time, conventional, and multiplex) | Identification of biofilm-producing genes |
| Method | Application | Objective |
|---|---|---|
| MtP | Microtiter plate: Biofilm produced on wells’ walls in response to agent | Agent measurement effects on biofilm production |
| MBEC | Minimum biofilm eradication concentration: Biofilm detection created on wells’ walls in response to agent and identifying agents MBEC | Agent measurement effects on the production of mature biofilms on wells’ walls |
| Vortex accompanied with plate counting | Plate counting of bacterial growth induced by biofilms and detecting MBEC agents | Screening antimicrobial agents’ activity against bacterial growth induced by biofilms |
| Checkerboard assay | Plate counting of bacterial growth induced by biofilms and calculating FIC indices | Antimicrobial activity screening of a combination of agents |
| Sonication accompany with vortex, and plate counting | Detecting biofilm produced on wells’ walls in response to agent and identifying MBEC agents | Screening the activity of antimicrobial agents against bacterial growth induced by biofilm |
| Quantitative PCR | Measuring specific biofilm gene expression | Gene expression induced by biofilm and monitoring expression in response to agents |
| MS | Mass spectrometry: Located exoenzymes measurement in biofilm matrix | Bacterial protein monitoring expression in response to agents |
| Devices | Substratum | Type of Culture | Screening Counting Method |
|---|---|---|---|
| Modified Robbins | Silastic disks | Batch | Viable (36) |
| Disk reactor | Teflon coupons | Batch | Viable or direct, after sonicating, vortexing, and homogenizing coupons (36) |
| Calgary biofilm | Plastic polycarbonate pegs | Batch | Viable, after sonicating of pegs (36) |
| Flow cell | Chambers with transparent surfaces | Continuous | Confocal laser scanning microscopy (36) |
| Perfused biofilm fermenter | Cellulose-acetate filters | Continuous | Viable, after shaking filters in sterile distilled water (36) |
| CDC biofilm reactor | Plastic connectors | Continuous | Growth medium continuous flow for biofilm formation of glass coupons by sonicating coupons individually (36) |
| Model bladder | Urinary catheters | Continuous | Direct examination by chemical analysis or SEM or TEM (36). |
| Nuclease-based fluorescence | - | - | Some indirect detection methods for example end-point staining methods by crystal violet in microtiter well plates or tubes to screen attached bacteria on extracellular polysaccharides target surfaces (56) |
| Fluorescence-based real-time screening | - | - | Direct investigation of new antibiofilm agents/surfaces in a continuous mode against biofilms (57) |
3.4. Therapeutic Approaches
Infection treatment associated with biofilm establishment requires further studies because of observing high levels of antibiotic resistance induced by biofilm structures. The concerned treatment is usually a mixed therapy, especially for infections induced by biofilm with macrolides such as clarithromycin, erythromycin, and azithromycin. These drugs display a wide range of anti-biofilm activities against infections because of the biofilm occurred by Gram-negative bacteria and prevent the production of alginate as a key matrix component (43).
It is documented that macrolides are effective against biofilms due to Staphylococcus spp. and P. aeruginosa (58). Moreover; it is proved that the utilization of clarithromycin together with vancomycin is effective in eradicating both planktonic cells and biofilm structures (59). The usage of roxithromycin with imipenem facilitates the better neutrophils influx into biofilm backbone and unfixes the biofilm (60). The use of catheters to coat and impregnate these antimicrobial drugs is another approach to solve the problems with biofilm production (61). Further, due to its bactericidal actions, silver has also been used to coat catheters. Silver encompasses broad-spectrum antimicrobial characteristics (62, 63). The variants of the synthetic cationic peptide derived from natural peptides have been used as a strategy to target biofilms. Recently, some substances with antibacterial properties (e.g., genuine, nitrofurazone, and nitrous oxide) have been used to alter the urinary catheters’ surface (43, 64).
Biofilm structure eradication is highly complicated due to the high levels of drug resistance presented by these constructions. Recent treatments are an alternative to medication with existing antibiotics to elude not only biofilm constitution but also resistant bacteria in underlying tissues. Table 9 presents a revision of some recent attitudes towards anti-biofilm treatments.
| Method | Function |
|---|---|
| Catheters covered with antibiotics or hydrogels | They are hydrophilic polymers making the catheter capable of increasing surface lubrication and consequently reducing the bacterial adhesion to this surface, thereby playing a role in decreasing the encrustation of catheters; An increase > 45% in CAUTI is perceived by silver alloy employment in a urinary catheter covered by hydrogel (65, 66); It is proved that catheters covered with minocycline-rifampicin stop biofilm formation in Gram-negative and Gram-positive bacteria, Candida spp. and even P. aeruginosa (67) |
| Antibiofilm treatments by nanoparticles | These particles can stick and penetrate into bacterial cells, damage the bacterial membrane, and act with chromosomal DNA(68); Biofilm formation blockage in E. coli and S. aureus strains by glass surfaces coating with MgF nanoparticles is observed. Yttrium fluoride (YF3) nanoparticles have low solubility, extended protection, and low cytotoxicity (69); CaO-NPs (microwave irradiated CaO nanoparticles) have the potential to inhibit the biofilm production of Gram-negative and Gram-positive bacteria (70); Silver nanoparticles are applied for coating medical devices because of the silver antibacterial attributes. These nanoparticles, as part of biosensors, have been used in medical and pharmaceutical nano-engineering for diagnostic approaches, transfer, and therapeutic agents (71). |
| Iontophoresis | Iontophoresis, a physical process in a medium where ions stream diffusively, applies as an experimental, diagnostic, and therapeutic method. It uses voltage gradients as a kind of transdermal drug delivery procedure. It was proved that low electrical currents increase he tactivity of tobramycin and biocides against biofilm producing P. aeruginosa (72); Iontophoresis inhibit biofilm formation. Electric current use to these catheters covered by silver electrodes remarkably decreased their formation (73, 74). |
| Biofilm-degrading enzyme | A new antibiofilm urease induced by P. mirabilis convert change urea to ammonium ions; fluorofamide can prohibit the enhancement in pH by Proteus mirabilis, thereby preventing the urea crystal formation and the subsequent encrustation and catheter blockage; vanillic acid (75), natural plum juice, and germa-γ-lactones (76) can forcefully prevent bacterial growth of crystal formation in catheters by inhibiting urease enzymes; DspB, an enzyme pertaining to the bacteriophage and extracted from Actinobacillus species, ruin a crucial adhesion essential for biofilm production in E. coli and Staphylococcus (77); c-di-GMP is the second messenger significantly conserved among bacteria. It decreases biofilm production by reducing c-di-GMP inside cells (78). |
| Antagonism among different bacteria | Antagonism can cause with using different E. coli avirulent strains (79, 80) such as E. coli, HU2117 strain originated from E. coli 83972. Avirulent strains can colonize persistently without any symptomatic infection (81); hence, it has been applied for urinary catheters to reduce of biofilm production by other pathogenic strains (80). In individuals with an intermittent catheterization, a decrease in the development of UTIs is reported. |
| Bacteriophages | Specifically, lytic phages (the natural predators of bacteria) can infect bacteria, interrupt the normal bacterial metabolism (especially the biofilms of P. mirabilis and E. coli), and support viral replication (82); phages of S. aureus (83) with bactericidal activity control biofilm formation by replicating at infection site and depolymerize the destruction of the biofilm EPS matrix (84, 85). |
| Quorum sensing (QS) inhibitors | QS is a kind of relationship among bacterial cells responding to extracellular signaling molecules, called autoinducers (AIs) (86). AIs, small signal molecules proportional to cell-density related to gene expression (87), can regulate several processes involved in virulence such as motility and biofilm formation (88). This is necessary for planktonic bacteria to present the biofilm phenotype. An effective QSI inhibitor should have some characteristics, as described below (89): A, capable of excluding gene expression pertaining to QS by a low molecular mass; B, significant specificity for QS regulators; C, No toxicity foreukaryotic cells, D, non-interference with the fundamental bacterial metabolic processes to elude the resistance development; E, chemically permanent, resistant to the host metabolism, and inhabiting in the host cell for a long time; HSL, N-acyl homoserine lactone, is analogous to the QS signal. It can emulates blocking QS, with signals to receptor binding, and prohibit the biofilm formation of S. aureus (90). Garlic extract can enhance the susceptibility against tobramycin by modifying the architecture of bacterial biofilms (91). Moreover, peptides display the QSI activity; The RNAIII-preventing peptide is capable of prohibiting agr-mediated biofilm production in drug resistant S. epidermidis (90, 92). |
| Low-energy surface acoustic waves (SAW) | SAW intervenes with planktonic microorganisms’ adhesion to cellular surfaces (88). It has been applied for urinary catheters to reduce of biofilm production by other pathogenic strains (93, 94). |
| Antiadhesive compounds/molecules | It should specifically interact with the adhesions of pathogen and prohibit the union among pathogen and eukaryotic cell. It decreases invasion or host cells infection and eludes reversion; Cranberry produces a proanthocyanidin trimer in the extract and has an anti-adherence effect against uropathogenic E. coli (UPEC). Other antiadhesion agents are pilicides, mannosides, and curlicides (43). |
4. Conclusions
About 80% of infectious diseases are induced by biofilms; hence, the ever-increasing proliferation of microbes against human-made medication and inherent capabilities such as biofilm production have expanded the challenges posed to medical sciences. In recent years, research efforts have focused on the progression of effective remedial techniques not generating drug resistance in microbial association. In this regard, understanding bioavailability mechanisms, the survival and development of microbes within the biofilm structure, and the role of this feature in the spread of chronic diseases is a key to discovering effective methods to deal with germs. Despite extensive research on microbial biofilms, there are still some ambiguities regarding their mechanism of action, which highlights the significance of conducting further studies.