1. Background
Brucellosis, as the most common zoonotic disease, is caused by Brucella species, which are Gram-negative coccobacilli. This disease is considered a serious threat to public health worldwide (1) and is endemic to Middle Eastern countries, especially Iran. Statistics show that the annual incidence of brucellosis, estimated at 1: 100,000 in Iran, is increasing (2, 3). People who consume unpasteurized dairy products or undercooked meat products, as well as those who have contact with livestock, are exposed to a higher risk of infection (4). Also, certain occupational groups, such as livestock breeders, butchers, slaughterhouse workers, veterinarians, and laboratory staff, are considered as high-risk groups (5).
The main clinical manifestations of brucellosis include fever, fatigue, arthralgia, muscle pain, night sweats, chills, weight loss, splenomegaly, and hepatomegaly, respectively (6). Brucellosis also causes complications in various systems, including the osteoarticular, nervous, urogenital, gastrointestinal, hematologic, and cardiovascular systems. The broad spectrum of the clinical manifestations of this disease makes it similar to other systemic and local diseases, resulting in delayed diagnosis or misdiagnosis (7). Considering the diversity of non-specific clinical manifestations of brucellosis, it is necessary to identify the risk factors and perform laboratory tests to diagnose the disease.
According to national guidelines, detection of agglutinating antibodies through Wright test and 2-mercaptoethanol (2ME) test, besides the assessment of clinical symptoms, has a high diagnostic value for brucellosis (8). The results of other laboratory tests are usually normal in this disease, although abnormalities in the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete blood count (CBC), and serum levels of liver enzymes have been reported (9). The World Health Organization (WHO) recommends the combination of oral rifampin with doxycycline for six weeks for the treatment of patients with acute brucellosis. Nevertheless, physicians may prescribe other antibiotic combinations. Despite the use of combination therapy and long-term treatment, treatment failure, and relapse may occur, which are major challenges in the treatment of brucellosis (10).
2. Objectives
This study aimed to identify the epidemiology, clinical and laboratory manifestations, and outcomes of brucellosis in patients. By increasing the available information about different aspects of brucellosis, we can help physicians and health officials in the treatment and management of this disease.
3. Methods
3.1. Study Design and Setting
In this cross-sectional study, brucellosis patients admitted to three hospitals affiliated to Shahid Beheshti University of Medical Sciences, Tehran, Iran, between April 2015 and September 2020, were included. This study was conducted at Loghman Hakim, Imam Hossein, and Shahid Labbafinejad hospitals.
3.2. Data Collection
The patients’ medical records were reviewed for epidemiological findings, clinical manifestations, laboratory and imaging findings, and treatments. The patients were followed-up by phone calls to determine the rates of relapse and treatment failure. The diagnosis of brucellosis was based on clinical manifestations, along with the results of confirmatory tests. The clinical manifestations included the symptoms and signs of brucellosis. The confirmatory tests included a positive serum agglutination test or positive culture. According to the national guidelines, titers of ≥ 1: 80 and ≥ 1: 40 on Wright and 2ME tests were considered positive, respectively (11). Also, ESR, CRP, CBC, and the serum levels of liver enzymes and bilirubin were extracted from the medical profiles of patients.
In this study, a white blood cell (WBC) count of 4,000 to 10,000 cells/µL was considered normal. Anemia was defined as a hemoglobin level < 13 g/L. Also, a platelet count between 150,000 and 400,000 cells/µL was considered normal. On the other hand, ESR > 30 mm/h, CRP > 10 mg/L, creatinine level > 1.5 mg/dL, AST > 40 U/L, ALT > 40U/L, ALP > 115U/L, and total bilirubin > 1.5 mg/dL were considered to be elevated. The osteoarticular complications were confirmed by imaging. Neurobrucellosis was confirmed by lumbar puncture and cerebrospinal fluid (CSF) analysis. Suspected cases of endocarditis were ruled out by echocardiography. Also, epididymo-orchitis was diagnosed by sonography.
3.3. Data Analysis
The obtained data were processed in SPSS version 18.0. For descriptive variables, frequency, percentage, mean, and standard deviation were reported. Qualitative variables were compared between the groups, using Pearson’s chi-square and binomial tests. One sample t-test was also used to analyze quantitative variables. P-value < 0.05 was considered statistically significant.
3.4. Ethical Considerations
This study was approved by the Ethics Committee of School of Medicine of Shahid Beheshti University of Medical Sciences (approval ID: IR.SBMU.MSP.REC.1399.142). The study was conducted in accordance with the Declaration of Helsinki (2000), and the patients’ information remained confidential.
4. Results
4.1. Demographic and Epidemiological Characteristics
A total of 104 patients were included in this study. The detailed demographic and epidemiological characteristics of the patients are shown in Table 1. Overall, 56 (53.8%) patients were male, and 48 (46.2%) were female; there was no significant difference between males and females (P = 0.493). The mean age of the patients was 43.07 ± 18.521 years (range: 2 - 81 years). There was a significant difference between different age groups in terms of frequency (P = 0.001). Most of the patients (n = 63, 60.6%) had a history of dairy consumption, 29 (27.9%) patients had contact with livestock, and 21 (20.2%) patients were livestock breeders. The results showed that 70 (67.3%) patients were in the acute phase of the disease, while 34 (32.7%) patients were in the chronic phase. The number of men was higher in the acute phase (P = 0.041). However, there was no significant difference between patients in the chronic phase in terms of gender (P = 0.121).
| Variables | Values |
|---|---|
| Gender | |
| Male | 56 (53.8) |
| Female | 48 (46.2) |
| Age group | |
| Mean age | 43.07 ± 18.521 |
| ≤ 20 years | 11 (10.6) |
| 21 - 40 years | 37 (35.6) |
| 41 - 60 years | 35 (33.7) |
| > 60 years | 21 (20.2) |
| Province | |
| Tehran | 53 (51) |
| Lorestan | 13 (12.5) |
| Hamedan | 7 (6.7) |
| Kurdistan | 5 (4.8) |
| Zanjan | 4 (3.8) |
| Others | 22 (21.2) |
| Risk factors over the past three months | |
| Dairy consumption | 63 (60.6) |
| Contact with livestock | 29 (27.9) |
| Travel to endemic regions | 8 (7.7) |
| Family history of brucellosis | 5 (4.8) |
| Occupation | |
| Livestock breeder | 21 (20.2) |
| Laboratory staff | 2 (1.9) |
| Veterinarian | 1 (1.0) |
| Others | 80 (76.9) |
| Duration | |
| Acute phase (< 3 months) | 70 (67.3) |
| Chronic phase (> 3 months) | 34 (32.7) |
Demographic and Epidemiological Characteristics of the Patients (n = 104)
4.2. Clinical Characteristics and Complications
Table 2 shows the clinical characteristics of the patients, including the symptoms, signs, and complications. The most common symptoms were fever (80.8%), chills (58.7%), backache (55.8%), and sweating (51%), and the most frequent sign was splenomegaly (15.4%). The most common complication was osteoarticular involvement (21.2%), followed by neurobrucellosis (6.7%). Also, 19 (18.3%) cases had a fever of unknown origin (FUO).
| Variables | No. (%) |
|---|---|
| Symptoms | |
| Fever | 84 (80.8) |
| Chills | 61 (58.7) |
| Backache | 58 (55.8) |
| Sweating | 53 (51.0) |
| Weight loss | 46 (44.2) |
| Fatigue | 45 (43.3) |
| Muscle pain | 43 (41.3) |
| Anorexia | 35 (33.7) |
| Peripheral arthralgia | 35 (33.7) |
| Headache | 32 (30.8) |
| Nausea | 25 (24.0) |
| Cough | 11 (10.6) |
| Abdominal pain | 11 (10.6) |
| Gait disorder | 11 (10.6) |
| Testicular pain | 8 (7.7) |
| Dysuria | 7 (6.7) |
| Blurred vision | 4 (3.8) |
| Loss of consciousness | 1 (1.0) |
| FUO | 19 (18.3) |
| Signs | |
| Splenomegaly | 16 (15.4) |
| Hepatomegaly | 3 (2.9) |
| Lymphadenopathy | 1 (1.0) |
| Complications | |
| Osteoarticular involvement | 22 (21.2) |
| Spondylitis | 12 |
| Sacroiliitis | 6 |
| Osteomyelitis | 2 |
| Local abscess | 2 |
| Neurobrucellosis | 7 (6.7) |
| Gastrointestinal involvement | 5 (4.8) |
| Cholecystitis | 4 |
| Pancreatitis | 1 |
| Epididymo-orchitis | 5 (4.8) |
| Skin presentations | 5 (4.8) |
| Macular rashes | 3 |
| Acnes | 1 |
| Petechial rashes | 1 |
| Endocarditis | 0 (0) |
Clinical Characteristics and Complications of the Patients (n = 104)
4.3. Laboratory Findings
Table 3 presents the hematological and biochemical findings of the patients. The most common abnormal hematological presentations were anemia (67.3%), leukocytosis (11.9%), and thrombocytopenia (11.1%). The mean hemoglobin level was 11.75 ± 1.66 g/L. There was a significant difference between the mean hemoglobin level and its lower limit of normal (P < 0.001). The mean leukocyte count and platelet count were within the normal ranges; there were significant differences between their mean values and the lower limits of normal (P < 0.001).
Among biochemical findings, elevated ALP (89.7%), elevated CRP (57.7%), and elevated ESR (48.1%) were the most common. There were significant differences between the mean ESR and CRP and their upper limits of normal (P < 0.001). Also, there was a significant difference between the mean ALP and its upper limit of normal (P < 0.001). Despite the higher mean levels of AST and ALT relative to the normal ranges, there were no significant differences between their mean levels and their upper limits of normal (P = 0.843 and P = 0.920, respectively). Table 4 presents the serological findings of the patients.
| Laboratory Test | Mean | Standard Deviation | Assumed Value | P-value |
|---|---|---|---|---|
| WBC × 109 cells/L | 7.59 | 3.11 | 4.000 | < 0.001 |
| Hb (g/L) | 11.75 | 1.66 | 13 | < 0.001 |
| PLT × 109 cells/L | 251.65 | 106.06 | 150.000 | < 0.001 |
| ESR (mm/h) | 43.90 | 37.66 | 30 | < 0.001 |
| CRP (mg/L) | 32.94 | 31.09 | 10 | < 0.001 |
| Cr (mg/dL) | 1.05 | 0.04 | 1.5 | < 0.001 |
| AST (U/L) | 38.67 | 62.92 | 40 | 0.843 |
| ALT (U/L) | 40.62 | 57.73 | 40 | 0.920 |
| ALP (U/L) | 271.63 | 197.17 | 115 | < 0.001 |
| Total bilirubin (mg/dL) | 0.82 | 0.59 | 1.5 | < 0.001 |
Hematological and Biochemical Findings of the Patients
| Variables | No. (%) |
|---|---|
| Wright test | |
| < 1.80 | 20 (21.1) |
| 1.80 | 16 (16.8) |
| 1.160 | 23 (24.2) |
| 1.320 | 16 (16.8) |
| ≥ 1.640 | 20 (21.1) |
| 2ME test | |
| < 1.40 | 23 (25.3) |
| 1.40 | 14 (15.4) |
| 1.80 | 20 (22) |
| 1.160 | 16 (17.6) |
| ≥ 1.320 | 18 (19.8) |
| Coombs Wright test | |
| < 1.40 | 16 (23.2) |
| 1.40 | 1 (1.4) |
| 1.80 | 8 (11.6) |
| 1.160 | 15 (21.7) |
| ≥ 1.320 | 29 (42) |
Serological Findings of the Patients
4.4. Treatments and Outcomes
The most common therapeutic regimens were doxycycline + rifampin (39.4%) and doxycycline + rifampin + aminoglycoside (32.3%). Table 5 presents the therapeutic regimens and outcomes of patients. Of 104 patients, 99 were followed-up successfully. The outcomes of three patients were unknown. Also, two patients died from causes unrelated to brucellosis. Of 99 patients successfully followed-up, 86 (86.9%) had improved clinical symptoms, 9 (9.1%) experienced treatment failure, and 4 (4%) showed relapse.
| Variables | Improvement | Failure | Relapse | Total |
|---|---|---|---|---|
| Doxycycline + aminoglycoside | 6 (6.1) | 0 (0) | 0 (0) | 6 (6.1) |
| Doxycycline + rifampin | 34 (34.3) | 3 (3) | 2 (2) | 39 (39.4) |
| Doxycycline + rifampin + aminoglycoside | 27 (27.3) | 4 (4) | 1 (1) | 32 (32.3) |
| Doxycycline + rifampin + ceftriaxone | 7 (7.1) | 0 (0) | 1 (1) | 8 (8.1) |
| Others | 12 (12.1) | 2 (2) | 0 (0) | 14 (14.1) |
| Total | 86 (86.9) | 9 (9.1) | 4 (4) | 99 (100) |
Therapeutic Regimens and Outcomes of Followed-Up Patients (n = 99) a
There was no significant relationship between the treatment regimen and patient outcomes (χ2 = 7.804, P = 0.800). Also, there was no significant relationship between the duration of disease (acute or chronic) and patient outcomes (χ2 = 6.336, P = 0.096). Similarly, there was no significant relationship between the clinical manifestations and patient outcomes. On the other hand, there was a significant relationship between osteoarticular complications and patient outcomes (χ2 = 23.274, P = 0.006). There was no significant relationship between other complications and patient outcomes.
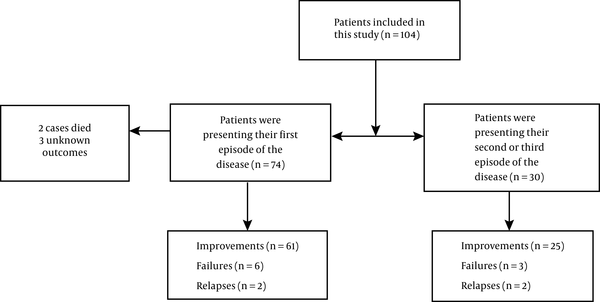
Based on the results, there was no significant relationship between patient outcomes regarding the episode of the disease (χ2 = 1.708, P = 0.721). The most common cause of treatment failure was the patient's poor compliance with treatment (84.6%). Side effects of antibiotic treatment were reported in nine patients, including 5 (4.8%) cases of nausea and vomiting, 3 (2.9%) cases of hearing loss, and 1 (1%) case of increased creatinine. Of 104 brucellosis patients included in this study, 74 were presenting their first episode of the disease, and the other 30 were presenting their second or third. Figure 1 shows the outcomes of patients in terms of the episode of the disease.
5. Discussion
Brucellosis is an endemic disease of Middle Eastern countries, especially Iran (3). This disease is a global health challenge with significant impacts on people and governments. Besides the significant financial burden on the healthcare system, it has a high burden of disease and reduces the quality of life of patients and those around them (12). Therefore, besides the importance of investigating various aspects of this disease, there is a need for efficient and comprehensive measures to control it (1).
In Iran, about 16,000 cases of brucellosis occur each year. The National Center for Infectious Diseases Management reported that from 1989 to 2013, the incidence of brucellosis decreased from 100 per 100,000 to 23.8 per 100,000. Brucellosis is present in all provinces of the country, but because of the occupation of livestock in the residents, it is more common in the Zagros mountains (13). The incidence of the disease shows a seasonal pattern in Iran so that it increases in spring and summer (because of mating and breastfeeding of livestock) and then decreases (14).
The epidemiological findings of the present study are consistent with previous research (15-18). The present results showed that the frequency of brucellosis was higher in men and the age group of 20 - 40 years. This finding is consistent with all previous studies, except one which reported that brucellosis is more common in patients over the age of 40 years. Also, about 23.2% of patients had high-risk occupations (livestock breeders, veterinarians, and laboratory staff), which is consistent with the literature. The high prevalence of brucellosis among young men, especially those engaged in livestock farming, may be due to the fact that in Iran, men are more involved in livestock farming than women (15, 16). Also, the use of personal protective equipment, such as gloves, goggles, and masks, is generally uncommon among high-risk occupational groups. Therefore, encouraging and recommending the use of protective equipment can be effective in reducing the incidence of brucellosis (17).
In this study, a significant number of patients had a history of consuming unpasteurized dairy products in the last three months, which is in line with all previous studies (15-18). It is known that the consumption of unpasteurized dairy products is the most important risk factor for infections caused by Brucellosis. Therefore, one of the most important challenges in controlling brucellosis in Iran can be the correction of wrong eating habits, such as using unpasteurized dairy products (16). Geographically, most patients in the present study were from Tehran Province and western provinces of the country. In a study by Bagheri et al., the highest prevalence of brucellosis was reported in western provinces of Iran, as these areas are the center of agriculture and livestock farming (16). The difference between the results of our study and those reported by Bagheri et al. may be due to the fact that the samples were not collected from a wide geographical area in this study, but instead, they were collected from only three referral hospitals located in Tehran.
Non-specific multi-systemic clinical manifestations of brucellosis can lead to misdiagnosis or delayed diagnosis of this disease and increase the number of chronic cases (19). In the present study, the most common symptoms and signs were fever and splenomegaly, respectively, which is in agreement with several previous studies (7, 18), but inconsistent with some others. Also, some studies reported fatigue (9) and arthralgia (20) as the most common manifestations, while fever was less common. Moreover, in some studies, the incidence of hepatomegaly was higher than splenomegaly (4, 20). Generally, both of these signs are attributed to the involvement of the reticuloendothelial system in brucellosis.
Furthermore, brucellosis causes complications in various body organs. The results of our study showed that the most common complications of brucellosis were osteoarticular involvement, neurobrucellosis, gastrointestinal involvement, and epididymo-orchitis. Sacroiliitis and spondylitis were the most common osteoarticular involvements, respectively, which is in line with a previous study (21). Also, one of the severe complications of brucellosis is neurobrucellosis, which had different manifestations in the present study, including impaired consciousness, headache, blurred vision, sensory and motor deficits, urinary incontinence, and epidural abscess. Previous studies have also reported these manifestations of neurobrucellosis. If neurobrucellosis is suspected, diagnostic and therapeutic measures must be taken as soon as possible (22, 23). Also, gastrointestinal involvement manifests as pancreatitis and cholecystitis. These complications have been rarely reported in patients with brucellosis (24, 25). Also, in our study, epididymo-orchitis presented with testicular pain and dysuria, as reported in previous studies (26).
The most common laboratory findings of the present study were anemia, normal WBC and PLT, elevated CRP and ESR, and elevated liver function tests (LFTs); these results are consistent with some previous studies (9, 18, 20). Thrombocytopenia and leukopenia have also been reported in a large number of patients with brucellosis (27); however, in our study, the frequency of leukocytosis was higher than leukopenia. This discrepancy may be attributed to differences in the cut-off values and populations of different studies. The most common laboratory test for the diagnosis of brucellosis is serology by standard tube agglutination (STA). In our study, 78.9% and 74.4% of the patients tested positive on the Wright and 2ME tests, respectively. The highest titers in the Wright and 2ME tests were 1.160 and 1.80, respectively. In a study by Nabavi et al., positive Wright and 2ME test results were observed in 90.7 and 55.1% of the patients, respectively (8). The cause of the difference in the 2ME results of these studies is probably the difference in the proportion of patients in the acute and chronic phases.
Generally, treatment of brucellosis is challenging due to medication side effects, long treatment periods, high frequency of treatment failure, and relapse. In the present study, the most common treatment was a combination of doxycycline and rifampin. In terms of patient outcomes, 87% of patients receiving the above treatment showed improvements. The recovery rate was almost the same for different antibiotic combinations. The combination of doxycycline with rifampin has been announced by the WHO as the first choice of treatment for brucellosis. However, a study by Jia et al. showed that in patients with osteoarticular complications, the combination of doxycycline with streptomycin increased the risk of relapse; therefore, it is suggested to use a three-drug therapy (9).
Moreover, the combination of doxycycline and rifampin with third-generation cephalosporins should be considered in the treatment of neurobrucellosis; treatment should continue for at least six weeks (28). According to our study, there was no significant relationship between the treatment regimen and disease outcomes. However, the study by Hasanjani Roushan et al. showed that the use of aminoglycosides (gentamicin or streptomycin) plus doxycycline was associated with a reduction in the relapse rate (29). The most common cause of treatment failure was poor compliance of patients with treatment (incomplete treatment). Therefore, instructions on how to use the drugs and explanations about the possibility of treatment failure if the treatment is not completed can be effective in reducing the failure rate.
This study has some limitations. First, brucellosis is a disease that is mostly treated on an outpatient basis, whereas our study population included hospitalized patients. Therefore, the results of this study may not be generalizable to all populations, as some cases included in this study were complicated and relapsed. Second, the sample size of this study was 104 people; a larger sample size would provide more generalizable results. Third, there was some missing data in the patients' laboratory tests due to a defect in the medical archives. Fourth, few studies have examined the outcomes and response to treatment in brucellosis patients, which made it difficult to analyze the disease outcomes and response to treatment.
5.1. Conclusion
The wide spectrum of non-specific clinical manifestations of brucellosis is a diagnostic challenge. Therefore, attention to epidemiological, laboratory and imaging findings can be helpful for physicians. Based on the present results, treatment failure in brucellosis was mostly due to the patient's poor compliance. Therefore, it is necessary to guide the patients on how to take their medications to improve the disease outcomes. Also, physicians should be well informed about the clinical and epidemiological characteristics of brucellosis. In endemic regions, brucellosis must be considered in the differential diagnosis of suspected cases, and laboratory tests must be performed to evaluate brucellosis.