1. Background
Coronavirus disease 2019 (COVID-19), brought about by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been well described as “a very visible pandemic” (1), affecting all aspects of global life and will be undoubtedly recorded as one of the top human health challenges in the 21st century. The unique aspects of this new infection have resulted in rapidly changing patient care strategies and protocols over time. These include high transmissibility of an airborne disease, distinct clinical manifestations, inadequate therapeutic strategies, and insufficient understanding of pathogenesis. Therefore, extensive studies are urgently needed to improve patient management in terms of prognostic tools and new therapeutic approaches. Up to the present, numerous investigations have assessed the efficacy of various biomarkers testing for COVID-19 to enable the world to respond to the challenge of severe cases of COVID-19 infection (2, 3).
Macrophage migration inhibitory factor (MIF), derived its name initially from the in vitro assay, is the protein secreted from lymphocytes and inhibits the macrophages from moving away from the protein release site. However, the name might be misleading because its role as a monocyte/macrophage chemotoxic factor has been well established (4-6). Initially, lymphocytes were considered the main cells secreting MIF. However, as previously reviewed (7), it became shortly uncovered that MIF is a pleiotropic cytokine secreted by a vast majority of cell types. The MIF is continuously synthesized and stored within intracellular storage pools (8).
The MIF secreting cells are vast cell types mainly consisting of lymphocytes, monocytes/macrophages, dendritic cells, blood granulocytes and mast cells, lungs epithelial cells, vascular endothelial cells, and endocrine glands or tissues involved in stress conditions, such as pituitary and adrenal glands (9, 10). These cells secret MIF as an important multifunctional factor in response to injury, inflammation, hypoxia, and other stress conditions and make a network of distinct responses, including upregulated innate and adaptive immune responses, and inflammatory responses (10, 11), and systemic quasi-hormonal response (12). The latter (stems emerging from) is linked to MIF endocrine regulatory role in systemic responses to severe stress conditions, such as endotoxic shock (13, 14). In addition to “multifaceted” immune and inflammatory signaling cascades (15, 16), MIF has been shown to exhibit an anti-glucocorticoid activity (17). The MIF is a double-edged sword. It is released to stimulate inflammatory responsespost-injury tissue healing activities (18); however, MIF release might result in uncontrolled acute inflammatory responses with tissue damage and organ dysfunction (19).
The role of MIF in numerous human diseases has been noted as reviewed by Lue et al. (20). It is worth mentioning that MIF changes have been observed in inflammatory diseases (21), pulmonary inflammation (22), lung injury (23), acute respiratory distress (24-26), septic shock (27), and endothelial damage (28). Interestingly, MIF inhibitor agents have been proposed for therapeutic purposes in septic shock and inflammatory conditions (8, 29).
2. Objectives
COVID-19 shares distinctive features with melanocyte-inhibiting factor activities; therefore, the present study hypothesized that this factor might be linked with COVID-19 severity and exacerbation. Considering the unique role of MIF as an early regulator of innate and adaptive immune responses, the current study aimed to analyze the serum level of MIF as a significant predictor of COVID-19 severity.
3. Methods
3.1. Study Design and Participants
This case-control study was performed in one of the primary university-affiliated centers devoted to COVID-19 subjects during November 2020 till April 2021. The research population included 60 patients with COVID-19 diagnosis as case group and 30 normal age- and sex-matched cases as control group. The infected cases were divided into two distinct groups, including 30 outpatients who had mild clinical symptoms and no pulmonary involvement in imaging tests and 30 patients hospitalized in the intensive care unit (ICU) who had respiratory distress syndrome (shortness of breath, oxygen saturation ≤ 93% at rest, respiratory rate ≥ 30 times/minute, and evidence of lung injury). The subjects were divided into mild and severe groups using the diagnosis and treatment protocol for patients with COVID-19 (trial version 8) (30).
The exclusion criteria of all groups consisted of main underlying conditions, like chronic heart disease, kidney and liver dysfunction, autoimmune disease, cancer, immunodeficiency, and receiving immunosuppressive, antiviral, or immune-boosting treatment. Electronic medical records were used to collect all clinical and paraclinical data. Fulfilling a minimum of one of the criteria, namely ICU admission, needed mechanical ventilation, and mortality due to any reason was described as a poor prognosis.
3.2. Laboratory Tests
The oropharyngeal and nasopharyngeal swabs of real-time polymerase chain reaction standard kits were used to find the SARS-CoV-2 viral nucleic acid. When the patients were admitted to the hospital, the blood samples were taken from all study groups. A complete blood count, C-reactive protein (CRP), hemoglobin (Hb), lactate dehydrogenase (LDH) activity, hematocrit (Hct), and serum MIF concentration were obtained in this study.
3.3. Assays for Serum MIF Measurement
Human MIF Quantikine Enzyme-Linked Immunosorbent kits (R & D Systems, USA) were utilized for the analysis of MIF serum levels. The quantitative sandwich enzyme immunoassay technique was applied in this assay. A human MIF- specific monoclonal antibody underwent pre-coating onto a microplate. Pre-diluted specimens and standards were added to the wells. After the washing process, a human MIF- specific enzyme-linked polyclonal antibody was integrated with the mixture. The next stage involved washing steps and adding the substrate to develop color. Color intensity was directly proportional to the bound MIF amount in the first step. Finally, the color development was stopped to measure the color intensity in the final products.
3.4. Ethical Considerations
We considered the ethical standards of the national and institutional research committees. Moreover, this research followed the Declaration of Helsinki on human research. The study approved by Ethics Committee of Mashhad University of Medical Sciences, (IR.MUMS.MEDICAL.REC.1399.562).
3.5. Data Analysis and Statistics
The descriptive indices like as mean and standard deviation and number and percentage were used for continuous and categorical data, respectively. The one way analysis of variance (ANOVA) and chi-square test were utilized for the comparison of the variables in the study groups. The Pearson/Spearman correlation tests were employed for the analysis of the possible relationships between variables as appropriate. Furthermore, the estimation of the prognostic value of serum MIF for COVID-19 patients was performed using the receiver operating characteristic (ROC) curve. The significance level was regarded as less than 0.05. The SPSS 16.0 and GraphPad Prism 6 were utilized for data analysis
4. Results
Table 1 describes the subjects’ characteristics. In this research, 58% (N = 52) of the patients were male. The subjects aged 25 - 68 years. Out of 30 severe cases, 5 patients passed away. More than half of the COVID-19 subjects in this study were middle-aged male patients over 30 years (Table 1).
| Variables | Mild Disease | Severe Disease | Healthy Controls | P-Value |
|---|---|---|---|---|
| Age, y | 38.7 ± 6.9 | 47.3 ± 10.3 | 39.5 ± 8.8 | 0.2 |
| Gender | ||||
| Male | 18 (60) | 17 (57) | 17 (57) | - |
| Female | 12 (40) | 13 (43) | 13 (43) | - |
| Fever | 8 (26) | 25 (83) | 0 | 0.01 |
| Cough | 8 (26) | 24 (80) | 0 | 0.006 |
| Fatigue | 14 (46) | 18(60) | 0 | 0.006 |
| Dyspnea | 4 (13.3) | 16 (53) | 0 | 0.001 |
| ARDS | 0 | 16 (53) | 0 | - |
| Death | 0 | 5 (16.6) | 0 | - |
| WBC (/µL) | 8350 ± 960 | 12303 ± 2090 | 7213 ± 645 | 0.006 |
| Neutrophils(/µL) | 6939 ± 942 | 11476 ± 2092 | 4071 ± 511 | 0.001 |
| Lymphocytes (/µL) | 1406 ± 124 | 827 ± 71 | 3124 ± 523 | 0.001 |
| NLR (%) | 4.5 ± 0.75 | 13.1 ± 2.9 | 1.3 ± 0.32 | 0.002 |
| Hb (g/dL) | 13.01 ± 1 | 12.7 ± 1.3 | 13.1 ± 1.4 | 0.24 |
| HCT (%) | 39.4 ± 2.8 | 38.6 ± 4.3 | 39.6 ± 4.4 | 0.31 |
| PLT (×103/µL) | 279 ± 37 | 210 ± 42 | 258 ± 40 | 0.05 |
| CRP (mg/L) | 33.7 ± 7.8 | 259 ± 80 | 3.26 ± 1.35 | 0.001 |
| LDH (U/L) | 235 ± 22 | 639 ± 173 | 217 ± 22 | 0.007 |
| MIF (ng/mL) | 40.45 ± 6.6 | 65.31 ± 6.2 | 20.63 ± 6.1 | < 0.0001 |
Abbreviations: WBC, white blood cells; NLR, neutrophil lymphocyte ratio; Hb, hemoglobin; HCT, hematocrit; PLT, platelet; CRP, C - reactive protein; LDH, lactate dehydrogenase; MIF, Macrophage migration inhibitory factor.
a Values are expressed as mean ± SD or No. (%)
4.1. Assessment of Serum MIF Levels in Study Groups
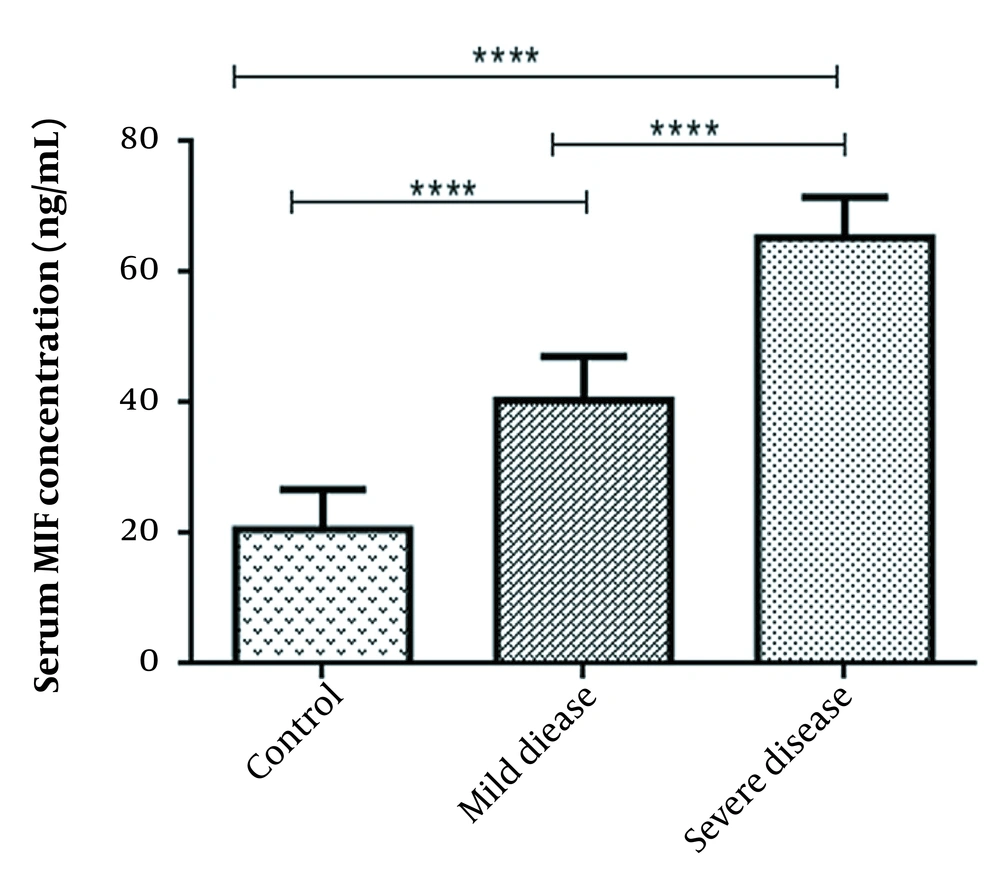
The subjects with severe COVID-19 demonstrated a higher serum MIF levels in comparison to those with mild symptoms (MIF mean value: 65.31 ± 6.2 and 40.45 ± 6.6 ng/mL respectively; P < 0.0001). Additionally, subjects with both mild and severe COVID-19 showed a statistically significant higher MIF level than the healthy individuals (MIF mean value for healthy subjects: 20.63 ± 6.1 ng/mL; P < 0.0001) (Figure 1).
Serum levels of MIF across study groups. ANOVA test was used to determine the differences, indicated by asterisks. The values show significant differences between control (N = 30) and severely-ill patients (N = 30, **** P = 0.0001), control and asymptomatic patients (N = 30, **** P = 0.0001), and between two groups of COVID positive patients (**** P = 0.0001).
4.2. Relationship Between Serum MIF and Clinical Characteristics
Based on the Pearson / Spearman correlation, in case group there was a strong positive correlation between MIF serum levels with Neutrophil Lymphocyte Ratio (NLR) and CRP. Although not statistically significant, higher MIF levels were also positively related to incidence of dyspnea, acute respiratory distress syndrome, and death in case group (P > 0.05; Table 2). However, lymphocyte count was inversely associated with MIF levels (Table 2). Moreover, no significant correlation was observed between MIF concentration with WBC, polymorphonuclear leukocytes (PMN), Hb, Hct, platelet count, LDH, fever development, and age and gender of participants (P > 0.05).
| Variable | Spearman/Pearson Value | P-Value |
|---|---|---|
| Lymphocyte | -0.85 | 0.01 |
| NLR | 0.89 | < 0.001 |
| CRP | 0.83 | < 0.001 |
| Dyspnea | 0.60 | 0.18 |
| ARDS | 0.53 | 0.55 |
| Death | 0.26 | 0.09 |
4.3. Prognostic Value of Serum MIF for COVID-19 Patients
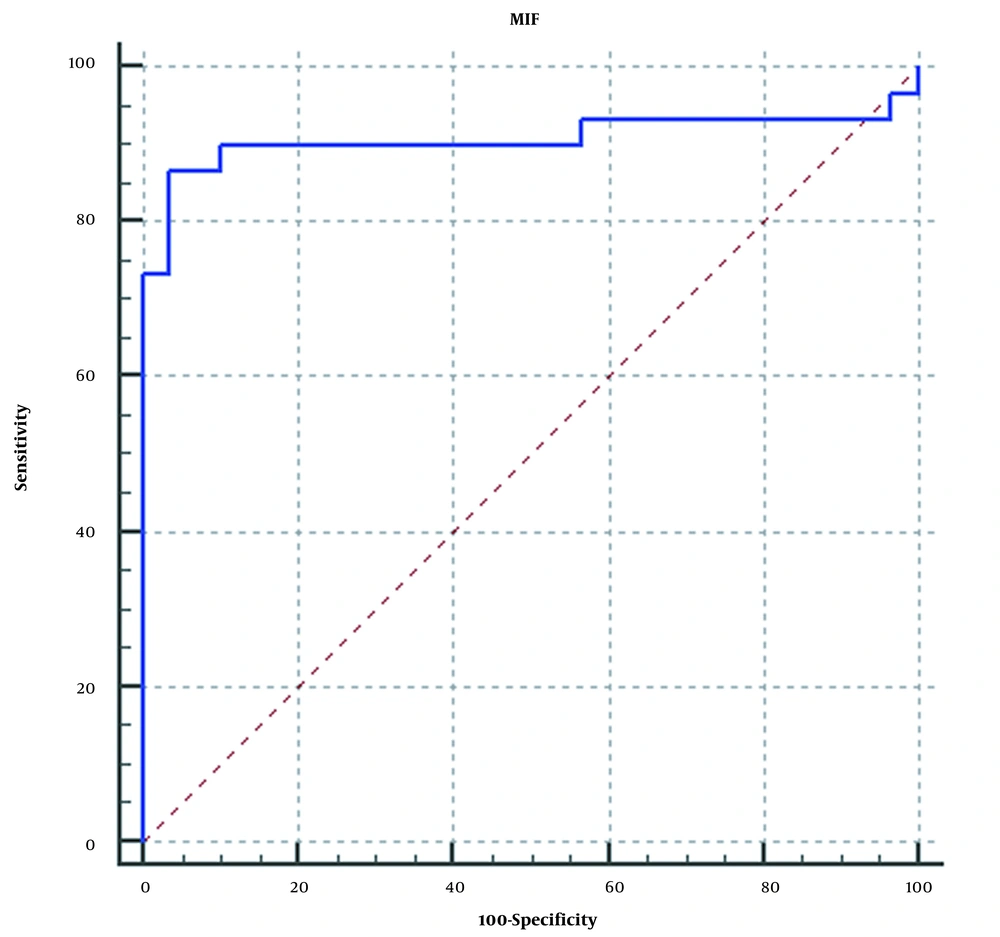
This study analyzed the prognostic performance of MIF as a marker of the COVID-19 severity. The area under the ROC curve (AUC) for cut-off point 50.7 ng/mL was 90.8% (95% CI: 80.5% to 96.7%, P < 0.001) (Figure 2). , and sensitivity and specificity were determined as 86.67% and 96.67%, respectively. This provides a positive likelihood ratio of 26 and a negative likelihood ratio of 0.14. Additionally, the results showed that significantly higher serum MIF levels were observed in patients with poor outcomes (58.38 [32.2-83.2 ng/mL] vs. 40.12 [27.1 - 55.8 ng/mL] for other patients) (P = 0.0001; Figure 2).
5. Discussion
This study showed an increase in MIF levels, along with COVID-19 disease severity. Greatly higher MIF levels were noticed in COVID-19 cases than in healthy individuals, especially in those who had severe complications (P < 0.0001). The second major finding was that severe cases of COVID-19 infection had significantly higher MIF levels than patients suffering from a mild disease (P < 0.0001). The aforementioned results suggest that there might be an association between MIF concentration and COVID-19 exacerbation; therefore, MIF can act as a marker of clinical severity for SARS-CoV-2 infection, particularly in case the clinical manifestations are insufficient to accurately predict disease progression. The MIF may act as a key mediator of systemic inflammatory responses in COVID-19 infection.
During the present study’s experimental work and data analysis, three publications on the topic became available. The current observations are consistent with the results of the aforementioned studies, indicating a prognostic role for MIF in COVID-19 patients. A study by Bleilevens et al. was carried out on 36 mechanically ventilated COVID-19 patients. The increased plasma levels of MIF were significantly associated with the development of organ dysfunction and significantly lower survival (31).
Another study by Aksakal et al. was performed on 110 patients diagnosed with COVID-19 and 40 healthy volunteers. Significantly, higher MIF levels were reported in the patients than in the controls. Furthermore, there was a higher level of MIF in severe patients than in moderate cases. The ROC curve analysis was carried out for the differentiation between severe and moderate COVID-19 subjects with MIF levels. The area under the curve was reported as 0.78. With the MIF cutoff value reported as 4.455 ng/mL, sensitivity and specificity were 83% and 62%, respectively (32). Similarly, Dheir et al. studied 87 COVID-19 patients, including 47 ICU-admitted and 40 ward-admitted patients. Regarding MIF levels, a significant difference was observed between the ICU and ward patients (P < 0.024). The authors also suggested that a MIF level > 4.705 is associated with a significantly increased risk of ICU admission (33). Like all patient-based studies, summative and supportive data from numerous centers are still needed to fulfill the knowledge gaps. In this regard, one possible argumentation is the cut-off values reported in the studies. The normal amount of MIF has been estimated to be up to 10 ng/mL in healthy individuals (34), though this estimation should be considered with serious caution. Indeed, the normal value of MIF has not been determined and such estimated values can be misleading. MIF amounts show a wide range of variation based on age (35). In addition, the MIF amount follows a circadian rhythm throughout the daytime (36). Notably, according to the manual of the ELISA kit used in the present investigation, the amount of MIF ranged from 15.3 - 52.3 ng/mL (mean 22.3 ng/mL) among 36 healthy individuals during kit development studies (available at: https://www.rndsystems.com). In fact, a wide range of variations has been observed in healthy control groups in different studies. In one meta-analysis study (37), the healthy controls serum levels ranged from 0.3 ± 0.012 up to 61 ± 58.7 ng/mL (38) based on the studies included in the meta-analysis. Also, in another meta-analysis (27), the mean serum levels of the control groups among different studies ranged from 0.121 ± 0.001 up to 46.829 ± 38.394 ng/mL (39). This wide range may arise from several factors such as technical issues or sudden release of MIF from the intracellular pool, therefore still huge studies are needed to determine a normal range for MIF concentration. The above-mentioned facts, every study can obtain a specific cut-off for the study, and the observed cut-off values cannot be generalized or included in patient care protocols; this may even be misleading in clinical applications. Nevertheless, still increasing or decreasing amounts of MIF has prognostic value.
The observed increased MIF levels among COVID-19 patients suggest a main pathophysiological role of MIF in the course of COVID-19 infection. Numerous aspects of pathophysiology and molecular network beyond COVID-19 infection have been fully discussed (40-42). In many of the proposed mechanisms, MIF might be a central molecule, including cytokine storm (43), innate (44, 45) and adaptive immune responses (46, 47), and inflammatory and antioxidant responses (48, 49). In particular, a key role of macrophages and their activators as central nodes of the network of events in COVID-19 patients has been fully noticed (50, 51). It has been demonstrated that MIF activates macrophages and plays specific roles in facilitating acute inflammatory responses through the promotion of the expression and secretion of several pro-inflammatory cytokines (i.e., tumor necrosis factor-alpha, interferon-gamma, interleukin 1 beta, interleukin 6, interleukin 8, macrophage inflammatory protein-2, cyclooxygenase-2, nitric oxide, and some products of the arachidonic acid pathway) (52).
Additionally, MIF stimulates T helper type 1 immune cell activity and amplification of macrophage functions, thereby regulating the production of acute-phase proteins, fever, and severe inflammation (52). This factor also counteracts the anti-inflammatory activity of glucocorticoids. Therefore, it seems reasonable that MIF might be a key point of COVID-19 pathogenesis. The MIF stands at the edge of several mechanisms involved in COVID-19 and gives a better picture of COVID-19 pathogenesis. The MIF exhibits a central role in tissue healing and pulmonary fibrosis (18, 53), chemotaxis, cytokine release, innate immunity, B cell and T cell activation, anticorticosteroid effect, vasculopathy, and other systemic and local responses. The main mechanisms by which MIF might be involved in severe conditions of COVID-19 patients remain to be fully discussed. Several biomarkers have been implemented in the management of COVID-19 (54); however, still additional effective diagnostic tools might help physicians to improve the clinical care of COVID-19 patients (55).
The MIF release and its prognostic value have been noted in other viral respiratory infections, such as influenza and respiratory syncytial virus (RSV) (56-58). In addition to lung damage, MIF has been blamed for being involved in vasculopathy and endothelial damage associated with the dengue virus (59). The increased levels of MIF have been correlated with prognosis and early mortality risk among septic shock patients (60).
Taken together, the results of the present study and previous investigations propose MIF as a biomarker in the management of COVID-19 patients. In addition, these observations highlight MIF in the pathophysiology of COVID-19. Meanwhile, due to the significant role of MIF in distinct pathways leading to disease exacerbation, clinical trial studies to investigate the possible therapeutic effects of MIF inhibitors and death prevention among severe COVID-19 patients might be beneficial. An additional uncontrolled factor is the possibility that nutrition status or medications could affect the results; however, the subjects with unfulfilled inclusion criteria (e.g., comorbidities, autoimmunity, and cancer) were ruled out to restrict the effect of confounding factors.
5.1. Conclusions
The current study and previous studies are consistent in increasing the amount of MIF during COVID-19 infection, especially among severe patients, and this can open one of the important and effective paths in COVID-19 pathogenesis and subsequently possible therapeutic approaches such as MIF inhibitors. However, it should be noted that a cutoff value could be misleading. Further studies are recommended to assess the pathophysiologic pathways in which MIF participates during COVID 19 and the effects of anti-MIF drugs on the improvement of patient conditions and reduction of mortality rates in possible upcoming peaks of infection.