1. Background
Pseudomonas aeruginosa (P. aeruginosa) has long been recognized as an opportunistic bacteria and a leading cause of severe hospital-acquired infections such as endocarditis, septicemia, urinary tract infection (UTI), ventilator-associated pneumonia (VAP), skin, surgical-site infections and eye and ear infections (1, 2). P. aeruginosa inflicts high morbidity and mortality rates, especially among infected burn patients (3, 4).
Treating infections caused by P. aeruginosa has become a worldwide challenge due to the potential of this bacterium to develop resistance to almost all available therapeutic agents (5). Unfortunately, the improper and irrational use of antibiotics has increased the prevalence of third- and fourth-generation cephalosporins and carbapenems-resistant strains (6, 7). It was previously thought that carbapenems were the last-line therapeutic drugs against multidrug-resistant (MDR) P. aeruginosa infections (8, 9). Carbapenem-resistant P. aeruginosa has become a considerable threat to public health worldwide (10). The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) consider carbapenem-resistant P. aeruginosa as one of the strains that require novel therapeutic approaches (10, 11).
Generally, acquired, intrinsic and adaptive resistance are the main coping mechanisms of P. aeruginosa against antibiotics (12). Carbapenem resistance of P. aeruginosa is due to a combination of factors facilitating the production of carbapenemases enzymes such as serine, carbapenemases, and metallo-ß-lactamases (MBLs) (New Delhi metallo-β-lactamase [NDM], imipenemase [IMP], and Verona integrin-encoded metallo-β-lactamase [VIM]), and deficient outer membrane porin D (OprD) (13). blaIMP and blaVIM genes are the most clinically important beta-lactamase classes. blaNDM-1 producing strains are resistant to a wide range of antibiotics and are becoming the foremost threatening carbapenemase (14, 15). The specific porin OprD facilitates the diffusion of small peptides, basic amino acids, and carbapenems into the cell (16, 17). The absence of OprD in P. aeruginosa leads to moderate resistance to imipenem (17, 18). These bacteria also produce plasmid-mediated enzymes that hydrolyze the oxyimino β lactams and the monobactams called expanded spectrum beta-lactamases (ESBLs). ESBLs confer a powerful resistance against ß-lactam antibiotics and third-generation cephalosporins such as ceftazidime, ceftriaxone, and cefotaxime inactive (19).
2. Objectives
The purposes of this study were to determine the antimicrobial susceptibility patterns and prevalence of MBLs, typing the isolates, and detect the oprD, blaCTX-M, blaSHV, blaTEM, blaIMP, blaNDM, and blaVIM genes among clinical isolates of P. aeruginosa collected from several hospitals of Tehran.
3. Methods
3.1. Ethics Approval
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences “IR.SBMU.MSP.REC.1400.481.” The patients’ personal information was not collected or included to maintain confidentiality.
3.2. Clinical Samples and Bacterial Identification
Eighty non-duplicate clinical isolates of P. aeruginosa were collected from children hospitalized in Tehran Hospitals from March 2019 to February 2020. Laboratory identification of isolates was performed by standard biochemical tests. All isolates were preserved in trypticase soy broth (Merck) supplemented by 20% glycerol at –70°C. P. aeruginosa ATCC 27853 was used for quality control.
3.3. Antimicrobial Susceptibility Testing
Antibiotic susceptibility of each strain to ceftazidime (30 µg), cefepime (30 µg), imipenem (10 µg), ciprofloxacin (5 µg), piperacillin/tazobactam (100/10 µg), amikacin (30 µg), gentamicin (10 µg), tobramycin (30 µg), were determined by the Kirby-Bauer disk diffusion, following the recommendations of the Clinical and Laboratory Standard Institute (CLSI) (20).
3.4. Screening of Metallo-β-lactamase
All P. aeruginosa isolates were evaluated for production of metallo-β-lactamase (MBL) by the combined disc diffusion test (21, 22), which was performed by imipenem and meropenem alone and in combination with ethylenediaminetetraacetic acid (EDTA) 0.5 M. A difference in zone diameter between discs alone and disc + EDTA 0.5 M ≥ 7 mm was explicated as a positive result for MBL production.
3.5. DNA Extraction
P. aeruginosa isolates were cultivated on Mueller Hinton Broth (Merck, Darmstadt, Germany) for 18 h at 37°C, and genomic DNA was extracted using the boiling method (23). DNA Concentration was evaluated by the Nanodrop instrument (WPA Biowave II Nanospectrophotometer, USA).
3.6. PCR Technique
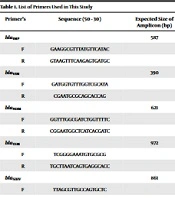
The presence of extended-spectrum β-lactamases and MBLs genes was examined using the primers presented in Table 1. PCR was executed in a final volume of 25 µL with 12.5 µL of Taq DNA Polymerase 2x Master Mix RED (Amplicon, Denmark; Cat. No.: A190303), including 1 × PCR buffer, three mmol/L MgCl2, 0.4 mmol/L dNTPs and 0.08 IU Taq DNA polymerase, one µL of 10 pmol of each primer and 7.5 µL of sterile distilled water. PCR program was set as follows: initial denaturation at 94°C for 5 min, followed by 36 cycles of 94°C for 1 min, and annealing at 54°C to 60°C, relevant to each primer for 45 s and final extension at 72°C for 5 min. The PCR products were electrophoresed on a 1% agarose gel.
| Primer’s | Sequences (5' - 3') | Expected Size of Amplicon (bp) |
|---|---|---|
| blaIMP | 587 | |
| F | GAAGGCGTTTATGTTCATAC | |
| R | GTAAGTTTCAAGAGTGATGC | |
| blaVIM | 390 | |
| F | GATGGTGTTTGGTCGCATA | |
| R | CGAATGCGCAGCACCAG | |
| blaNDM | 621 | |
| F | GGTTTGGCGATCTGGTTTTC | |
| R | CGGAATGGCTCATCACGATC | |
| blaTEM | 972 | |
| F | TCGGGGAAATGTGCGCG | |
| R | TGCTTAATCAGTGAGGCACC | |
| blaSIHV | 861 | |
| F | TTAGCGTTGCCAGTGCTC | |
| R | GGTTATGCGTTATATTCGCC | |
| blaCTX-M | 544 | |
| F | TTTGCGATGTGCAGTACCAGTAA | |
| R | CGCTATCGTTGGTGGTGCCATA | |
| OprD | 1329 | |
| F | ATGAAAGTGATGAAGTGGAG | |
| R | CAGGATCGACAGCGGATAGT | |
| RAPD | CCGCAGCCAA |
List of Primers Used in This Study
3.7. Random Amplified Polymorphic DNA Fingerprinting
Random amplified polymorphic DNA (RAPD-PCR) was conducted for all samples in accordance with Vanerkova described method (24). The total volume of the PCR reaction was 25 µL, which included 12.5 µL of 2x Master Mix, 5 µM of primer, and 1 µL of DNA extract. Thermal cycling was conducted with an initial denaturation at 94°C for 15 min, followed by 25 cycles of 94°C for 1 min, 55°C for 2 min, 63°C for 10 min, and concluded by a final extension of 65°C for 18 min. The PCR productions were loaded on the 1.5% agarose gel, and the results were analyzed.
4. Result
4.1. Patients and Bacterial Isolates
A total of 80 P. aeruginosa isolates were collected from hospitalized children in Tehran Hospitals. Among the 80 isolates obtained, 26 were from boys, 54 were from girls, and all were between 2 and 15 years old.
4.2. Antibiotic Susceptibility Profile
The resistance rate of P. aeruginosa isolates to the tested antibiotics was 28 (35%) to cefepime, 20 (25%) to ceftazidime, 18 (22%) to ciprofloxacin, 13 (16%) to tazobactam, 12 (15%) to gentamicin, 8 (10%) to tobramycin and amikacin. Out of 80 isolates, 16 (20%) were carbapenems-resistant.
4.3. Metallo-β-lactamase
Phenotypic and genotypic assessment of MBLs showed that among 16 isolates that were resistant to imipenem, four could produce MBL genes.
4.4. Prevalence of Resistance Genes
The existence of OprD, blaCTX-M, blaSHV, and blaTEM genes was detected in 80 (100%), 36 (45%), 22 (27.5%), 17 (21.25%), and 1 (1.25%) blaIMP, blaNDM genes, respectively. In this study, the blaVIM gene was not detected in the isolates, and no mutation was observed regarding the presence of an insertion element in the OprD gene (Table 2).
4.5. Random Amplified Polymorphic DNA Fingerprinting
All 80 P. aeruginosa isolates were subjected to random amplified polymorphic DNA (RAPD-PCR) fingerprinting to appraise the genetic diversity. Isolates were partitioned into 13 common types (CT) and 14 single types (ST).
5. Discussion
P. aeruginosa is an opportunistic pathogen and one of the main causes of acquired and nosocomial infections with limited therapeutic options. Furthermore, treating infections caused by this bacterium is extremely difficult and challenging (25, 26).
Antibiotic susceptibility investigation in this study demonstrated that the resistance levels amongst P. aeruginosa isolates as follows: cefepime (35%), ceftazidime (25%), ciprofloxacin (22%), tazobactam (16%), gentamicin (15%), amikacin (10%), and tobramycin (10%). The high prevalence of resistance rate to beta-lactam agents in this study was related to the presence of ESBLs, such as blaTEM, blaSHV, and blaCTX-M. Results demonstrated that nine blaCTX-M and blaSHV-positive ones could be identified among ceftazidime-resistant strains. Nonetheless, 15/28 cefepime-resistance isolates were ESBL producers. Therefore, additional resistance mechanisms or enzymes such as ESBLs, AmpC, and MBLs may have played a role in the resistance. In the study conducted by Shahbazzadeh et al., approximately 50% of isolates were resistant to third-generation cephalosporins. Among 51 isolates resistant to ceftazidime, 5 were ESBL producers (27). Moreover, Ali et al. showed that in 23.91% and 6.52% of samples, ESBLs and MBLs were detected, respectively (28).
According to the obtained results, aminoglycosides demonstrated low rates of resistance. This is consistent with the findings by Ahmadian et al. (2021), who similarly reported low levels of P. aeruginosa resistance to amikacin (13%), gentamicin (32%), and tobramycin (33%) in various clinical cases in Iran (29). Khan and Faiz also reported that low proportions of P. aeruginosa isolates were resistant to gentamicin (11.6%) and amikacin (7.4%) in Saudi Arabia (30). These low resistances against amikacin and tobramycin may be due to their lower prescription in Iranian hospitals. Similarly, it was reported in Kashfi et al.'s study that amikacin was more effective than gentamicin against P. aeruginosa isolated in burn patients (31). In contrast, the resistance rate to aminoglycosides is high in some regions and countries, and some authors believe that P. aeruginosa tends to show intrinsic resistance to aminoglycosides (32). For instance, in India, the resistance rates to amikacin, gentamicin, and tobramycin were 50%, 67%, and 66%, respectively (33). Generally, based on antibiotic resistance studies, it can be concluded that the rates of resistance in P. aeruginosa isolates are higher than before, which may be due to different reasons such as the difference in resistance mechanism, the irresponsible use of antibiotics in prevention, the difference in sample type and the topographical locations, and hygienic condition and hospitals care (34).
Carbapenems such as imipenem and meropenem are effective antimicrobial agents against P. aeruginosa infections. However, carbapenem resistance is emerging worldwide. Among the present study's 80 clinical isolates of P. aeruginosa, 20% were resistant to carbapenems. The resistance range of P. aeruginosa to imipenem in other studies in different regions was 5.5 to 62.5% (25, 35-38). Resistance to carbapenems in P. aeruginosa occurs via several mechanisms. Producing carbapenemase, the main mechanism of carbapenem resistance in Iran, is one of the main mechanisms (39).
Acquired MBLs such as IMP, NDM, and VIM are the most common, first detected in the early 1990s (40, 41). These genes are carried by genetic elements, including plasmids and integrons (42). PCR assays targeting carbapenemase and MBL encoding genes were negative in all 16 isolates. However, MBL was positive in 4 isolates’ phenotypic assay. Kalluf et al. reported 85.5% positive strains with phenotypic results for MBL (43). blaIMP and blaNDM were detected in one out of 28 cefepime-resistant strains. Our data support the findings of Shariati et al. from Iran, which demonstrated that MBL and carbapenemase were negative in carbapenem-resistant strains (4). It seems that the resistance to imipenem in these bacteria depends on mechanisms other than the production of carbapenemase enzyme. As reported in other studies, the frequency of MBLs was approximately 8.2 to 35.1% (44-46). Contrary to some reports, VIM was the predominant MBL gene associated with the outbreaks due to MBL-producing P. aeruginosa (47, 48).
The deficiency of OprD due to substitutions, deletions, insertions, or mutations have also been considered as another mechanism of carbapenem resistance (49). In the current study, PCR assay using the OprD - particular primers demonstrated that all isolates were positive for OprD amplification. Still, none of the imipenem-resistant P. aeruginosa isolates harbored an insertions element in the OprD gene. As shown by several studies, alteration of OprD increases the MIC for imipenem but not other carbapenems (38, 50). A different study by Wolter et al. showed the presence of IS elements within the OprD gene, which was not observed in this study (51). Kiani et al. reported one base IS element among five resistance strains (25). An investigation Performed by Shen et al. revealed that 136 out of 141 (96.5%) of the resistant isolates had mutations. Among them, only 6 strains had IS, and the remains had other types of alteration (50). These reports are contrary to our results. It is possible that mutations or deletions occurred in our strain. Various IS elements have been recognized worldwide that may inactivate the OprD gene, such as ISPpu21 and ISPa1328 in Iran, ISPa26 in South Africa, ISPa46 and ISPa1328 in France, ISRP10 in Croatia, ISPa133 in Spain, ISPa1328 and ISPre2 in China, ISPa150 in Russia, and ISPa8, ISPa1635, and ISPa1328 in the USA (4, 52-54). RAPD-PCR was performed for typing all P. aeruginosa isolates. In typing results, 13 CT and 14 different ST were detected. Notably, all carbapenems-resistant isolates were clonally related. Different distributions of genotypes have been shown in several studies. For example, Vaez et al. had 54 clinical isolates with 39 different groups, and Salimi et al. observed eight different groups from 29 isolates (55, 56). The difference in the obtained results may be due to the difference in the sources of P. aeruginosa that can lead to colonization of the host.
5.1. Conclusions
Resistance to most anti-pseudomonal antibiotics has become an emerging issue worldwide. Carbapenem resistance is a critical problem that develops due to several mechanisms. To sum up, we analyzed the MBLs and ESBLs production in P. aeruginosa strains. The findings of this study revealed that the prevalence of carbapenem-resistant strains among hospitalized children is increasing. We identified that most of the isolates were harboring ESBL genes. On the other hand, the results demonstrated that there was beta-lactam antibiotic resistance and clonal spread among hospitalized patients. This indicates the necessity of molecular surveillance in tracking beta-lactamase-producing strains.