1. Background
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19, is responsible for one of the most widespread and deadliest pandemics in human history. This pandemic has affected different aspects of human health and has imposed significant burdens on the global economy. Severe acute respiratory syndrome-coronavirus 2 is an enveloped RNA virus that belongs to the subgenus Sarbecovirus, genus Betacoronavirus, and the family Coronaviridae (1). Several SARS-CoV-2 variants have been characterized during this pandemic (2). It seems that the hospitalization rate of young children and infants infected with the last dominant and most heavily mutated variant was higher than that of individuals infected with other variants (3), probably due to its higher transmissibility rate. In addition, the risk of COVID-19 mortality is higher in pregnant women (4).
The transplacental transfer of maternal IgG antibodies to an unborn baby is a specific adaptive mechanism for providing protection against some pathogens (5), especially viruses and bacterial toxins. Although, according to several studies, either maternal COVID-19 infection or vaccination can result in the transplacental transfer of SARS-CoV-2-specific antibodies (6-17), different parameters, such as the altered glycosylation profile of antibodies and timing of vaccination/infection during pregnancy, may affect the efficiency of transplacentally acquired immunity (13, 15, 16). Additionally, the lower transplacental transfer ratio of specific and/or total IgG has been reported in certain areas around the world (18-20).
Moreover, previous studies have indicated the differential efficiency of the transplacental transfer ratio in distinct IgG subclasses (21). It may be attributed to the binding affinity to the neonatal Fc receptor (FcRn), which is strongly expressed in the endosomal vesicles of placental syncytiotrophoblasts (22). On the other hand, reports suggest that the serum IgG subclass concentrations are influenced by ethnicity (23). Thus, ethnicity may affect the transplacental antibody transfer efficiency.
2. Objectives
This study aimed to assess the placental transfer ratio of antibodies in Iranian seropositive women. Further attempts were also made to assess the effects of some maternal and neonatal variables on the transplacental antibody transfer efficiency.
3. Methods
3.1. Study Population
From September 2021 to November 2021, pregnant women presenting to Kamali Hospital in Karaj, Iran, were enrolled in this monocentric study. Maternal venous blood and cord blood samples were collected directly after delivery. The census method was used, and all 119 paired samples were collected from Iranian seropositive pregnant women. The most salient inclusion criteria for eligible pregnant women were a SARS-CoV-2 seropositive result and the ability to understand oral information (in Persian). The participants' demographic characteristics, including maternal age at delivery, maternal education, maternal weight (body mass index [BMI]), maternal blood group, neonatal sex, and neonatal birth weight, are reported in Table 1.
| Demographics/Characteristics | Values |
|---|---|
| Mothers (n=112) | |
| Maternal age at delivery (y); mean ± SD (range) | 30.9 ± 6.7 (13 - 47) |
| Maternal education; No. (%) | |
| Illiterate | 10 (8.9) |
| < Grade 12 | 44 (39.3) |
| High school | 42 (37.5) |
| Academic education | 16 (14.3) |
| Maternal BMI, mean ± SD (range) | 26.6 ± 4.4 (17.1 - 41.5) |
| Maternal blood group; No. (%) (missing, n = 21) | |
| A | 35 (38.5) |
| B | 19 (20.9) |
| AB | 6 (6.6) |
| O | 31 (34) |
| Neonates (n = 119) | |
| Neonatal birth weight (g); mean ± SD (range) | 3023.4 ± 562.8 (900 - 42,000) |
| Neonatal sex, No. (%) | |
| Male | 56 (47) |
| Female | 63 (53) |
Abbreviation: BMI, body mass index.
3.2. Antibody Measurements
The concentration of anti-SARS-CoV-2 IgG in the maternal and cord blood samples was measured using the EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG) kit, according to the manufacturer’s instructions. The results were considered protective if the OD ratio exceeded 0.8. If the corresponding ratio exceeded the upper limit of the analytical measurement range, the result was reported as >10. Based on the transplacental antibody transfer ratios, the participants were divided into 2 groups: transfer ratio ≤ 1 (n = 53) and transfer ratio > 1 (n = 66).
3.3. Statistical Analysis
Correlations between the maternal and cord blood antibody concentrations were evaluated using Pearson's correlation test, with the corresponding r and P-values. The transplacental antibody transfer ratio was calculated as the cord blood antibody concentration divided by the maternal antibody concentration. The chi-square test and t-test were used as appropriate to compare the demographic characteristics, antibody levels, and transplacental transfer ratios. The level of statistical significance was set at P < 0.05. Analysis was performed using STATA version 16.1 (StataCorp, College Station, TX, USA).
4. Results
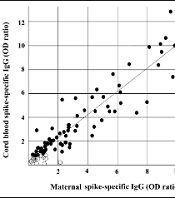
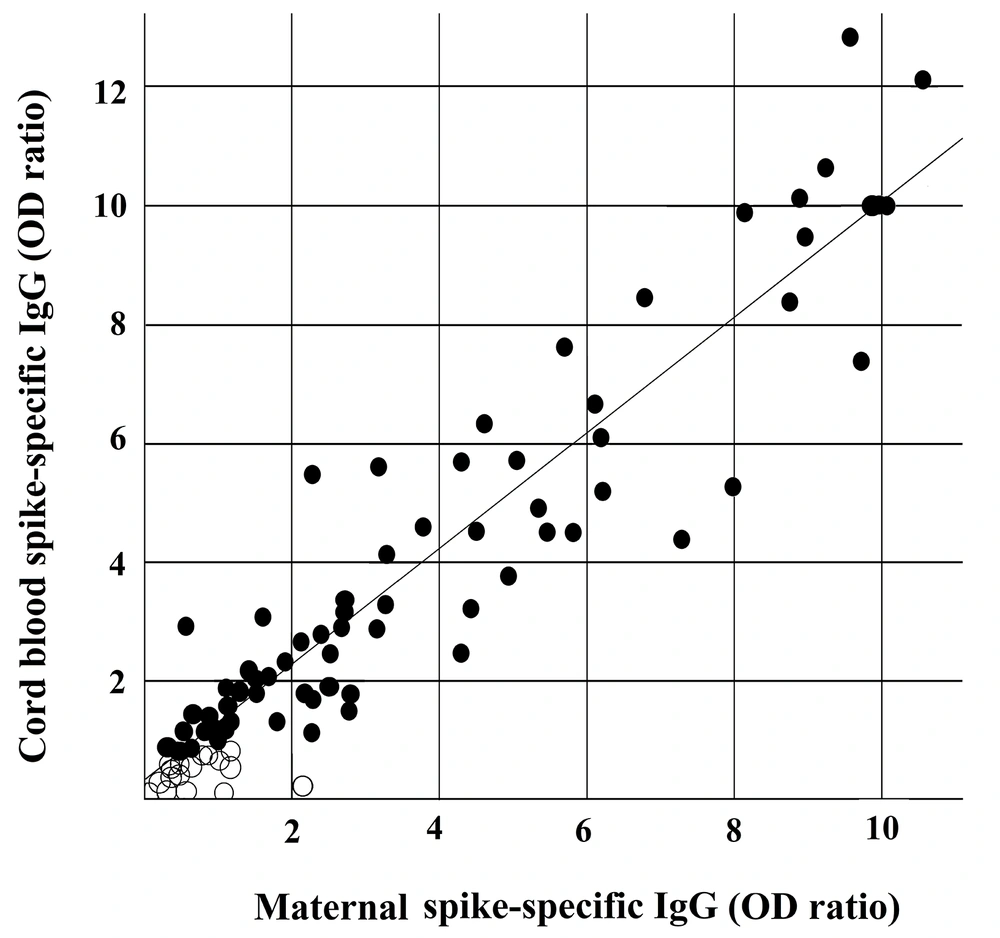
As shown in Figure 1, strong positive correlations were found regarding the concentration of SARS-CoV-2-specific IgG antibodies between the maternal plasma and cord blood samples in all pairs (r = 0.9646; P < 0.05), indicating the adequate level of transplacental transfer antibodies.
Correlations between maternal and matched cord blood IgG concentrations. Each dot represents data from a single pair of maternal and cord blood antibodies to the anti-spike domain of the S protein of SARA-CoV-2 (r = 0.9646; P < 0.05). Protective levels (> 0.8) are represented by filled circles, and insufficient protective levels (≤ 0.80) are represented by open circles.
The antibody concentrations in neonates born to mothers with high antibody titer levels also increased. Among all maternal-cord blood pairs of Iranian seropositive women, only 5% had a very low transfer ratio (< 0.5). Efficient transplacental transfer ratios (transfer ratio > 1) were observed in 55.46% of cases.
As reported earlier, the participants were divided into 2 groups based on the transplacental antibody transfer ratio (ratio ≤ 1 [n = 53] and ratio > 1 [n = 66]). Some variables, including maternal age at delivery, maternal BMI, neonatal birth weight, and neonatal sex, were compared between the 2 groups (Table 2).
| Characteristics | Transfer Ratio | ||
|---|---|---|---|
| ≤ 1 (n = 53) | > 1 (n = 66) | P-Value a | |
| Maternal age at delivery (y); mean ± SD | 30.9 ± 6.7 | 30.9 ± 6.6 | 0.95 |
| Maternal BMI; mean ± SD | 27.7 ± 5.1 | 25.7 ± 4.3 | 0.02 b |
| Neonatal birth weight (g); mean ± SD | 2874.5 ± 826.4 | 3138.5 ± 573.8 | 0.04 b |
| Neonatal sex; No. (%) | 0.30 | ||
| Male | 28 (52.8) | 28 (42.5) | |
| Female | 25 (47.2) | 38 (57.5) | |
Abbreviation: BMI, body mass index.
a P-values for continuous variables are determined by the t-test, and P-values for categorical variables are determined by the chi-square test.
b P < 0.05.
There was a significant difference in the transplacental transfer ratio regarding neonatal birth weight and maternal BMI (P < 0.05). Conversely, maternal age and neonatal sex did not affect the placental antibody transfer.
5. Discussion
The EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG) assay was used to analyze spike-specific antibodies collected from maternal and cord blood samples upon delivery. Previous studies, based on comparisons with the gold standard assay (microneutralization), have shown that this serological kit is suitable for evaluating protective immunity following infection or vaccination (24, 25). This standardized, commercially available kit is based on a serological test to detect specific anti-spike (S1) antibodies. It is known that the spike protein mediates viral attachment and entry into target cells (1). It is a viral fusion protein that contributes to the merging of the viral envelope with the cell membranes, allowing for the release of viral genomes into the cell cytoplasm (1).
In this study, spike-specific SARS-CoV-2 antibodies were measured in 119 maternal-cord blood pairs. The results showed that the level of protective SARS-CoV-2-specific antibodies in newborns depended on the concentration of maternal antibodies, as reinforced by the results of several other studies in different parts of the world (6-17). Besides, relatively efficient transplacental transfer ratios (transfer ratio > 1 in 66 out of 119 subjects [55.46%]) were observed in Iranian seropositive women. Similarly, a previous study examining 72 seropositive mother-infant pairs from 4 other race/ethnicity groups reported an efficient transplacental IgG transfer (transfer ratio ≥ 1) in 55.5% of cases (8). Notably, the transplacental transfer ratios (range, 0.8 - 1.7) were similar to those reported for vaccine-induced antibodies to other pathogens, such as influenza and pertussis (26, 27). The present findings highlighted the importance of vaccination during pregnancy to improve neonatal immune responses to SARS-CoV-2.
Currently, several other vaccines, such as tetanus toxoid, tetanus diphtheria acellular pertussis diphtheria (Tdap) vaccine, inactivated influenza vaccine, inactivated polio vaccine (IPV), pneumococcal polysaccharide vaccine, meningococcal polysaccharide vaccine, hepatitis A vaccine, hepatitis B vaccine, and inactivated rabies vaccine, are administered for routine use or under special circumstances to protect women and their offspring by placental transmission of maternal antibodies. Alternatively, infected/vaccinated pregnant women may transfer protective antibodies to newborn infants via breastfeeding after birth. Specific SARS-CoV-2 IgA antibodies have also been detected in the breast milk of seropositive mothers (28). Moreover, the detection of specific antibodies in the neonates' blood confirmed the acquired passive immunity (29-32).
The results of this preliminary study showed significant differences in the transplacental transfer ratio with respect to the neonatal birth weight and maternal BMI. It has been previously observed that low-birth-weight neonates have lower passive total and/or specific IgG levels because of an impaired transplacental transfer (33, 34). Moreover, a previous study showed that maternal obesity could affect the turnover of placental cells, such as syncytiotrophoblasts (35). Overall, the IgG transfer from the mother to the offspring occurs across syncytiotrophoblasts (5). Accordingly, maternal BMI may affect the efficiency of transplacental antibody transfer. However, further extensive studies with a larger sample size are needed to confirm the significant association between these variables and the transplacental transfer efficiency of specific SARS-CoV-2 IgG antibodies.
To the best of our knowledge, only 1 recently published study in Iran has examined the levels of maternal and cord-blood antibodies. However, this study only involved 23 pregnant women who received single or 2 doses of the BBIBP-CorV (Sinopharm) vaccine (36). Based on reports, Iran has reported millions of confirmed COVID-19 (98% recovered cases and 2% deaths). Until today, millions of COVID-19 vaccine doses have been administrated in Iran, and approximately most of the population is fully vaccinated. Additionally, pregnant women were not excluded from the vaccination program in Iran. Therefore, many newborns in Iran, especially those born to infected and/or vaccinated mothers, may acquire some maternal antibodies. However, future research needs to concentrate on the durability of passively acquired immunity in Iranian neonates.
5.1. Conclusions
Although there were significant differences in the transplacental transfer ratio regarding the neonatal birth weight and maternal BMI, more extensive investigations with a larger sample size are needed to confirm the significant association of these variables with the transplacental transfer efficiency of specific SARS-CoV-2 IgG antibodies. The present study supports previous studies conducted worldwide, demonstrating that vaccination during pregnancy is highly effective in enhancing neonatal immune responses against SARS-CoV-2.
5.2. Limitations
This study has several limitations. The most significant limitation is its small sample size. Therefore, further studies with a larger sample size are needed to confirm the significant association between variables and the transplacental transfer efficiency of specific SARS-CoV-2 IgG antibodies. Additionally, no demographically similar seronegative pregnant women were included as a control cohort. Last but not least, performing assays at different times might have affected the measurement of antibody levels.