1. Context
Cholera outbreaks are caused by the bacterium Vibrio cholerae, serotypes O1 or O139. The disease manifests as sudden severe watery diarrhea that could lead to serious dehydration and death if not treated with oral or intravenous hydration solutions. Vibrio cholerae is easily transmitted via the fecal-oral route and can rapidly spread to multiple communities. In more severe cases, it can cross national borders and cause a far-reaching epidemic surge. We are currently witnessing an unprecedented rise in cholera epidemics, evident in the 29 countries reporting cholera outbreaks during 2022, compared to fewer than 20 countries reporting them throughout the last five years (1, 2). The urgency of the situation is further underscored by the shift in vaccine strategy from a standard 2-dose regimen to a one-dose approach due to vaccine shortages. The global disease trend indicates more numerous, severe, and widespread outbreaks, necessitating stronger prevention and treatment interventions. Herein we highlight the recent epidemiology, diagnosis, and treatment alternatives that could help combat this persistent plague.
1.1. Epidemiology
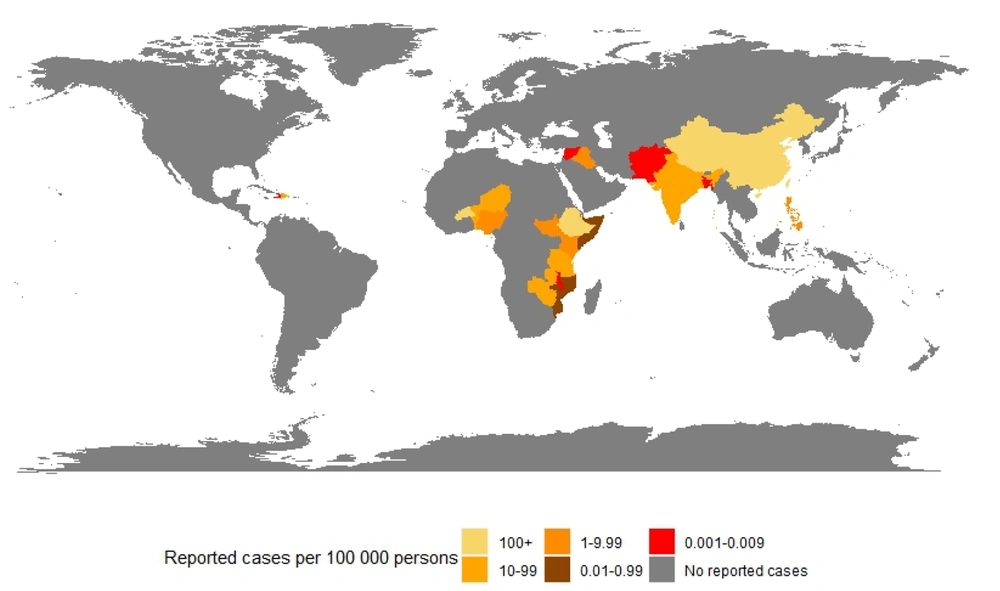
The earliest recorded outbreak of cholera dates back to 1817 in the Ganges Delta of India (3). Since then, cholera has spread through trade routes and caused seven different pandemics between 1817 and 1961, with the last still ongoing and affecting countries in Africa, Asia, and South America (3). According to the latest World Health Organization (WHO) estimates, 2.86 million cholera cases occur annually in endemic countries, with 95,000 dying from the disease. However, precise estimates remain challenging due to the lack of standard reporting of cholera cases and deaths and the lack of healthcare access in countries affected by wars, such as Yemen and Syria (1). This is particularly important, considering that 84% and 41% of all cases and deaths linked to cholera were in Yemen in 2017 (2). Based on data from the European Centre for Disease Prevention and Control, countries with 100 or more reported cases per 100,000 persons between January 2022 and January 2023 included Afghanistan, Bangladesh, Lebanon, Malawi, Pakistan, and Syria (4). Figure 1.
1.2. Transmission and Risk Factors
Vibrio cholerae, the causative agent of cholera, is primarily transmitted through the ingestion of contaminated food or water, although person-to-person transmission can occur. According to the World Health Organization (WHO), the majority of cholera cases are associated with contaminated water sources, such as wells, rivers, and lakes, or with food prepared with contaminated water. This is evident in the quality of drinking and domestic water sources and springs in cholera-prone Ugandan communities, which were found to be outside the WHO-recommended values (5). Household hygiene can be an important factor in mitigating cholera transmission, especially with recent evidence suggesting that interventions targeting case-centered and within-household transmission are most effective (6).
Vibrio cholerae thrives in certain environmental conditions, including alkaline pH, temperatures up to 30 degrees C, and 15% salinity, often found in brackish water in estuaries and coastal regions. Climate events such as rainfall can impact the dynamics of cholera spread. For instance, the El Niño phenomenon, characterized by the warming of surface water in the eastern and central equatorial Pacific Ocean, caused rainfalls and floods and was linked to the emergence of cholera in specific districts of Uganda (7). Nevertheless, drought- and famine-affected areas were also struck by cholera. This rather complex dichotomy was observed in Niger, where cholera surged during severe droughts in 2004 and resurged in 2006 following excessive rainfalls (8). Moreover, the disruption of sanitation systems, as in countries affected by wars, has resulted in cholera outbreaks. These countries include the Democratic Republic of the Congo, Somalia, South Sudan, Sudan, Syria, Yemen, and Zimbabwe (9, 10). Crowded camps and slums, where open defecation is common and pit latrines are scarce, are also high-risk areas for cholera outbreaks.
Host factors also play an essential role in determining the risk of V. cholerae infection and its symptoms. Lower socioeconomic conditions and extremes of age are frequently associated with higher infection risk (11). Other host factors impacting infection risk and severity include diet and immunity. Malnutrition has been shown to be associated with an increase in the duration of diarrhea and hospitalization. Furthermore, protein-energy malnutrition reduced the protective efficacy of an orally administered cholera vaccine in a mouse model (12). Protection against cholera can be provided by breastfeeding due to breast milk antibodies and glycans, which have been shown to exert a vibriocidal immune response and reduce the risk of severe cholera (13).
Unexpectedly, reduced host immunity, as seen in people suffering from Acquired Iimmunodeficiency Syndrome (AIDS), did not affect the severity of cholera but might be associated with a higher risk of infection. This was exemplified in Port-au-Prince, Haiti, where the prevalence of HIV infection in patients with culture-confirmed cholera was four times higher than the adult prevalence in the region (14).
1.3. Pathogen and Pathogenesis
Vibrio choleraeis a motile, gram-negative, rod-shaped bacterium belonging to the Proteobacteria phylum. Vibrio choleraehas more than 200 serogroups that vary in virulence, epidemiology, and evolutionary lineages. The serological classification of cholera strains is based on differences in the sugar composition of the heat-stable surface somatic "O" antigen. In fact, the majority of V. cholerae serogroups are not pathogenic, with only two groups, 'O1' and 'O139', being associated with cholera epidemics and pandemics (15). The two biotypes of V. cholerae O1, namely classical and El Tor, have distinct roles in cholera epidemiology. While the classical biotype is associated with the first six pandemics, the ongoing seventh pandemic is attributed to the El Tor biotype (16).
The infectious dose of these V. cholerae species varies depending on a multitude of host, pathogen, and environmental conditions. Animal models show different infectious doses when compared to human studies, with the latter requiring doses of 108 - 1011 to produce consistent colonization. Despite acid resistance mechanisms, adding a bicarbonate buffer to neutralize gastric acidity has been found to reduce the infectious dose in humans (17).
Vibrio cholerae is known to be acid-resistant, utilizing an acid tolerance response (ATR) when exposed to human gastric acids (18). The ATR mechanism of V. cholerae involves several physiological changes that allow the bacterium to maintain its structural integrity and metabolic activity in low pH environments. For example, V. cholerae alters its gene expression, enhancing the expression of the lysine decarboxylase, CadA, under conditions of low pH and high lysine concentrations. CadA, among other amino acid decarboxylases, consumes protons in their enzymatic reactions, thus maintaining internal pH (19).
Similar to environmental pH, the presence of bile acids represents a major component in virulence regulation for V. cholerae among other enteropathogens (20, 21). ToxR, a transmembrane transcription factor, possesses a periplasmic domain serving as an environmental sensor for bile acids (22). Positioned within a regulatory cascade, ToxR triggers the expression of toxin coregulated pilus (TCP) and cholera toxin (CT) (23). CT, housed within the cholera toxin bacteriophage (CTXϕ), falls under the direct control of ToxT (24). Research on the impact of bile acids has yielded mixed results, with some studies suggesting a repressive effect on ToxT-dependent transcription of CT and other virulence factors (25, 26). Conversely, bile acids have been shown to induce ToxR and CT transcription through a ToxT-independent mechanism (27, 28).
The influence of bile acids may also be subject to modulation by calcium concentrations. In the presence of established bile acid inducers of tcpA, the pilus subunit, a notable increase in tcpA expression was observed with elevated calcium levels, while this effect was mitigated upon calcium chelation within murine intestines (29). Another abundant molecule in the intestines is bicarbonate, which has been observed to enhance ToxT binding affinity to virulence gene promoters when at high levels, thus serving as an in-vivo signal modulator, particularly given its high concentration near the epithelium (30). In summary, V. cholerae adeptly responds to environmental cues like gastric acids, bile acids, and calcium, modulating its virulence and pathogenesis.
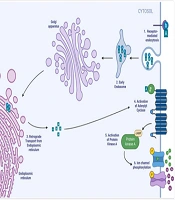
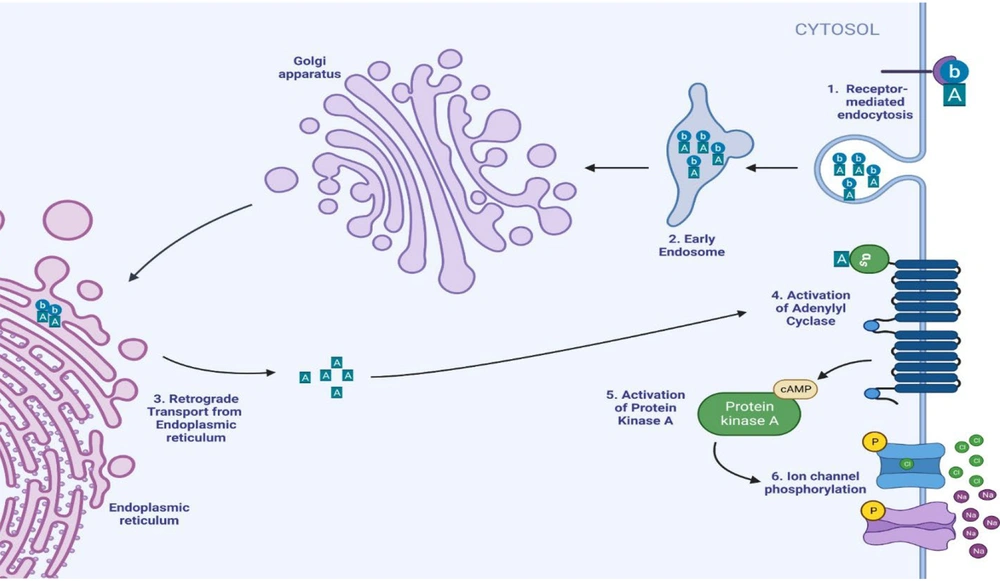
Vibrio cholerae leverages flagellar motility to penetrate the mucus layer and establish intestinal colonization. The complex transcriptional changes that follow are mediated by the production of ToxT. TCP is encoded in the V. cholerae pathogenicity island 1 (VPI-1) and plays a crucial role in attaching V. cholerae to human intestinal Caco-2 cells (31) (Figure 2). CT, encoded in the cholera toxin bacteriophage (CTXϕ), comprises a single A subunit (CTA1) and five B subunits (CTB1-5) arranged hexamerically. Subunit B binds to the ganglioside GM1 cell surface receptor on human jejunal epithelial cells, entering the cytoplasm through receptor-mediated endocytosis and retrograde transport from the endoplasmic reticulum (32). The A subunit catalyzes ADP ribosylation of adenylate cyclase (AC), leading to increased AC activity and intracellular cAMP concentration. Elevated cAMP activates protein kinase A (PKA), which phosphorylates the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), enhancing chloride secretion into the intestinal lumen (33). A single intraperitoneal injection with CFTR inhibitors belonging to the Thiazolidines chemical class reduced cholera-induced fluid secretion in mice by more than 90% over 6 hours (34). Another target of the CTA-PKA pathway is the inhibition of the Na+/H+ exchanger 3 (NHE3), thus increasing Na+ in the intestinal lumen (35). The combination of elevated sodium and chloride expands luminal fluid volume, resulting in watery diarrhea.
Another facet of cholera pathogenesis includes the formation of microcolonies and biofilms, along with quorum-sensing signaling systems and regulatory networks. Vital proteins for these processes feature the type IV pilus and TCP among others. Microcolony and biofilm formation are favored in the environmental conditions of low cell density, which are enhanced by CT-induced augmentation of luminal fluid volume. The low cell density is also crucial for pathogenicity and immune evasion as high cell density induces quorum sensing that activates HapR, a transcription factor. Regarding pathogenicity, HapR binds to promoters to repress the expression of CT and TCP. Regarding immune evasion, HapR reduces bacterial tryptophan uptake, thus providing host enterocytes with precursors for serotonin synthesis that activate innate immune signaling (36). In brief, V. cholerae exploits flagellar motility, ToxT-mediated transcriptional changes, TCP, and CT to colonize the intestine, induce diarrhea, and evade immune detection through microcolony and biofilm formation.
1.4. Laboratory Diagnosis
Diagnosing cholera presents challenges due to the similarity of symptoms with many other causes of gastroenteritis. Limited facilities and supplies, especially in underdeveloped areas where cholera is prevalent, exacerbate these challenges. Traditional culturing methods for diagnosis are time-consuming, which is problematic given the rapid and severe nature of cholera infections that demand prompt intervention. Consequently, clinical diagnosis is often relied upon during diarrheal illness outbreaks. Additionally, laboratory findings such as hypokalemia, hypocalcemia, metabolic acidosis, and isonatremic dehydration can provide supporting evidence for cholera before confirmatory tests are reviewed (37).
As the recent harsh cholera outbreaks inspired the current review, we will first shed light on effective diagnostics in such settings. Rapid diagnostic tests (RDTs) are considered a valid method for an initial alert of a cholera outbreak. Antibody-based cholera RDTs work by detecting V. cholerae's lipopolysaccharides (LPS) within 15 to 30 minutes in a stool sample. In a meta-analysis involving 20 studies and 8 distinct commercial rapid tests, the combined sensitivity relative to bacterial culture, the gold standard for diagnosis, was 90% (95% CI, 86 - 93), with a specificity of 86% (95% CI, 81 - 90) (38). The results generated by RDTs are not as specific and sensitive as those generated by polymerase chain reaction (PCR), bacterial culture, or darkfield microscopy (39). However, some studies found RDTs' accuracy, particularly those with enrichment in the alkaline peptone water (APW) step, comparable to that of stool culture when using PCR as a reference (40).
Efforts should be placed into improving RDTs to make them effective point-of-care (POC) testing tools in developing countries that are susceptible to recurrent cholera outbreaks and lack sufficient microbiological laboratories and expertise.
1.5. Clinical Features
Depending on the inoculum size and the individual's susceptibility, the incubation period of cholera ranges from several hours to three to five days. The most distinctive clinical feature of cholera is acute watery diarrhea. As cholera may be confused with other diarrheal diseases, severe cholera, also called cholera gravis, stands out with its characteristic profound and rapid loss of fluids, which typically has a fishy odor. Fluid loss may reach as high as one liter per hour in adult patients and 20 mL/kg/h in children (41). Another unique feature of severe cholera diarrhea is the passage of profuse rice-water stool (42).
The resultant hypovolemia is the most lethal sequela of the diarrhea, which may manifest as hypotension, tachycardia, dry mucous membranes, dizziness, decreased urine output, and in more severe conditions, shock, and death. This was evident in the early stages of the cholera epidemic in Haiti when the median time between the onset of symptoms and death in individuals who died before presentation to a cholera treatment center was 12 hours (43). Other gastrointestinal manifestations of cholera infection are abdominal cramping and vomiting, which may begin before or after the onset of diarrhea.
Significant complications of this illness include metabolic acidosis, which may occur due to the loss of stool bicarbonate or lactic acidosis from poor perfusion. In addition, pneumonia may occur due to vomiting accompanied by aspiration. The latter is considered frequent comorbidity in children with a high mortality rate (44).
1.6. Treatment
1.6.1. Fluid Replacement
Replacing lost fluids and electrolytes constitutes the cornerstone of cholera treatment. The main method of achieving this is through oral rehydration solution (ORS). The currently utilized WHO standard ORS formulation, established in 2002, is glucose-based reduced osmolarity (sodium 75 mEq/L, glucose 75 mmol/L, and osmolarity of 245 mOsm/L), as sodium is better absorbed when glucose is present (32). Rehydration takes place in two steps: Replacement and maintenance. Through clinical assessment and WHO guidelines, the degree of dehydration and the amount of fluids needed are determined (45).
Preferably, fluids are administered as ORS rather than intravenously due to lower costs, less invasiveness, and fewer emergency department revisits (46). However, in the case of profound ongoing stool losses, termed high purging (≥ 15 mL/kg per hour; seen in 3 - 5% of patients), failure of ORS attempts, and severe dehydration, intravenous fluids become indicated (47, 48). Neutral amino acids were found to increase the intestinal potential to absorb sodium and water ions, but there is insufficient evidence to qualify them as a standard ORS therapy (49). On another note, a review of thirty-five trials showed that patients treated with rice-based ORS experienced fewer and shorter diarrhea bouts than their glucose-based ORS-treated counterparts (50).
1.6.2. Antibiotics and Antibiotics Resistance
When treating cholera with moderate to severe dehydration, antibiotic administration becomes warranted to (1) reduce the time and severity of the disease by up to 50% and (2) limit the transmission of the viable organism to 1 - 2 days (51). Antibiotic administration comes after the initial fluid deficit is replenished, typically in about 4 hours. Antibiotics are chosen according to the patient’s condition and the antibiotic resistance pattern.
Antibiotic resistance is a major obstacle in the treatment of V. cholerae infection. The mechanisms of antibiotic resistance development include the overuse and misuse of antibiotics in both human medicine and the animal industry, efflux pumps, genetic mutations, and horizontal gene transfer (52). A recent meta-analysis showed that previously utilized bacterial cell wall inhibitors, such as aztreonam, cefepime, and imipenem, remain efficient with almost non-existent resistance (53). Other available antibiotic options that could work against cholera include tetracyclines, doxycycline, fluoroquinolones, and macrolides.
Tetracyclines remain a primary choice in treating cholera infection. They have comparable outcomes with doxycycline in terms of stool output, duration of diarrhea, and the requirement for ORS, according to a study in Bangladesh (54). However, high resistance to these two classes has been observed, requiring their use to be limited to settings where ongoing surveillance shows most strains are susceptible to those classes. Consistently, the high use of ciprofloxacin due to its superior effectiveness compared to tetracyclines has led to a dramatic rise in fluoroquinolone resistance (55). Regarding macrolides, azithromycin and erythromycin have shown clinical and bacteriological efficacy. In some instances, azithromycin was superior to fluoroquinolones, yielding better clinical outcomes (56, 57).
Considering the geographic variation in V. cholerae antibiotic resistance patterns is vital. A recent meta-analysis revealed varied rates based on geography, with 0% resistance to novobiocin and ofloxacin in Africa, gatifloxacin and levofloxacin in Asia, and ciprofloxacin in North America, thus necessitating the monitoring of regional and local antibiotic resistance patterns and the use of derived treatment guidelines (58).
1.6.3. Vitamins and Minerals
According to WHO recommendations, a 14-day course of zinc supplementation for children aged 6 months to 5 years can aid in shortening the duration of diarrhea. This is supported by the results of a meta-analysis including 33 trials (59). Contrary to WHO guidelines of 20 mg per day, recent research suggests that half the dose has a lower risk of vomiting but comparable efficacy (60).
Additionally, vitamin A supplementation is warranted when deficiency symptoms accompany acute diarrhea. Furthermore, in resource-limited areas, the routine administration of vitamin A has been associated with reduced morbidity and mortality (61).
1.7. Prevention
The prevention of cholera outbreaks heavily relies on the development of safe and effective water sanitation systems. The biggest limitation to the development of these systems is the high capital cost and extensive resources required to build the infrastructure. A living example of the effectiveness of these infrastructure upgrades in preventing cholera outbreaks is London in the 1800s, where the pioneering epidemiological work of John Snow was followed by the design and construction of a system for sewage disposal (62). In fact, Target 7c of the United Nations' Millennium Development Goals was to halve the proportion of the population without sustainable access to water and basic sanitation by 2015. Despite significant progress in that capacity, an estimated 1.8 billion people worldwide still drink water from sources that are fecally contaminated (63).
Since V. cholerae is transmitted via a fecal-oral route, the importance of water, sanitation, and hygiene (WASH) services extends to personal hygiene and cooking practices. Intriguingly, when compared to water quality or excreta disposal, hand washing with soap was more effective and reduced diarrheal disease by 42 - 48% (64). For cooking practices, Quick et al. found a seven-fold higher risk of illness in people eating cold cooked or raw seafood in El Salvador (65).
Another pillar in the prevention of cholera outbreaks includes early and rapid disease detection and treatment. As mentioned in the diagnosis section, RDTs combined with APW have an 89% sensitivity and 98% specificity that can help with surveillance efforts and medical resource management to limit the severity of outbreaks (39).
Further reduction of cholera risk was attained when oral cholera vaccines (OCVs) were administered along with improved WASH systems in endemic settings (66). This was supported by Malembaka et al.'s finding of single-dose OCV effectiveness of 44.7% 24 - 26 months after vaccination compared to controls (67). The two types of oral cholera vaccines (OCVs) include killed whole-cell vaccines and live attenuated vaccines. Regarding WC vaccines, three are prequalified by the WHO: Dukoral®, Shanchol®, and Euvichol-Plus® (56). Dukoral® contains killed whole cells of the O1 strain along with the recombinant B subunit of CT and provided negative protection in children under 5 years and 15% protection in children over 6 years of age within one year of surveillance. Shanchol® is bivalent, containing killed whole cells from both O1 and O139 V. cholerae strains, and provided 45% protection in all age groups and only 17% in children under 5 years within one year of surveillance (68).
The other type of OCVs is live-attenuated vaccines, including Vaxchora®, which is the only FDA-approved cholera vaccine for travelers aged 18 - 64 years. CVD 103-HgR I (Vaxchora®), composed of O1 strains that have been genetically modified to remove the gene encoding the CTA subunit (the toxic subunit), has 90.3% protective efficacy 10 days after vaccination and 79.5% after three months. However, it has no proven efficacy in endemic settings (69).
Current WHO recommendations include the use of OCVs in cholera-endemic areas, as well as for people at high risk of cholera during outbreaks. However, OCVs should not be seen as a replacement for other preventive measures, such as improving access to safe water and sanitation and promoting good hygiene practices (62).
2. Results and Conclusions
The ongoing cholera epidemic, driven by V. cholerae, is a critical global health concern. The disease's severe watery diarrhea and rapid transmission through contaminated sources lead to dehydration and potential death if not promptly treated. Cholera outbreaks are increasing, affecting multiple countries, and necessitate urgent preventive measures. Improving water sanitation, promoting hygiene practices, and implementing early detection are crucial for prevention. Vaccination with oral cholera vaccines offers a promising intervention. Timely fluid replacement and appropriate antibiotic therapy are essential for effective treatment. Combating this escalating epidemic requires immediate and concerted global efforts to save lives and prevent further spread.