1. Context
There is a growing body of evidence indicating that microbial organisms in the gut, collectively referred to as the microbiota, play a crucial role in the metabolic processes of their host (1). These processes include the activation of vitamins (2), support for immune function, and maintenance of intestinal health (3). Recent studies have also highlighted the association between the microbiota and various diseases, such as cardiovascular diseases (4), insulin resistance, obesity, and autoimmune diseases (5, 6), among others.
Furthermore, research has shed light on the role of choline metabolism pathways carried out by the gut microbiome in contributing to the pathogenesis of various disorders, as observed in both animal and human studies (7). Choline, a primary component of phosphatidylcholine, undergoes metabolism by the gut microbiome, resulting in the formation of an intermediate compound known as trimethylamine (TMA). Trimethylamine is subsequently oxidized within the intestine by microorganisms or transported to the liver, where hepatic flavin monooxygenases oxidize it further to produce trimethylamine N-oxide (TMAO). While TMAO was previously considered a waste metabolite with no significant biological effects, recent evidence strongly suggests an association between TMAO and inflammatory mechanisms (8, 9), atherosclerosis (10), thrombosis (11), and various pathological conditions.
Moreover, it has come to light that TMAO may increase the expression of scavenger receptors (SRs) (12). Among these receptors, SR-B1 is of particular interest as it has multifunctional roles, including facilitating the entry and efflux of cholesterol esters derived from high-density lipoproteins (HDL) into cells and tissues. Interestingly, SR-B1 has also been implicated in the entry of different viruses, including SARS-CoV-2, into host cells (13). A recent study by Wei et al. suggested that SR-B1 may enhance the uptake of SARS-CoV-2 and be associated with disease severity (12). These findings raise the possibility of an association between the presence of SR-B1 and the severity of COVID-19.
At the end of 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in China, leading to the emergence of COVID-19 (14). The virus quickly spread globally, and in March 2020, the World Health Organization (WHO) declared it a pandemic. Since then, over 29.5 million individuals have contracted COVID-19 worldwide, resulting in approximately 1 million deaths (15). COVID-19 manifests with a range of symptoms, from mild to severe respiratory tract diseases, often accompanied by serious complications affecting various organs, potentially leading to organ failure and fatalities. Approximately 20% of COVID-19 patients develop severe complications, including acute immune responses characterized by the overproduction of inflammatory cytokines, commonly referred to as a cytokine storm, followed by respiratory distress syndrome (16). Other significant complications include hypercoagulation, increasing the risk of thrombosis, and digestive symptoms, such as diarrhea, which have been observed in some patients (17).
Numerous investigations have explored the relationship between gut microbiome metabolites and COVID-19. A recent study conducted in China revealed a positive correlation between the levels of Lactobacillus species in the gut and a more favorable prognosis in COVID-19 patients. This association was linked to an increase in the levels of interleukin-10 (IL-10) (18). Conversely, some pro-inflammatory species, such as Klebsiella, Streptococcus, and Ruminococcus gnavus, have been found to promote the production of pro-inflammatory cytokines, potentially exacerbating the severity of COVID-19 (19). Additionally, Esposito et al. documented changes in the gut microbiome of children with Kawasaki disease compared to healthy children. In their study, they observed higher levels of Streptococcus species relative to Lactobacillus, indicating dysbiosis in the gut microbiome of children with Kawasaki disease (20). These findings highlight the importance of understanding the relationship between specific metabolites and compositions produced by the gut microbiome and the pathogenesis and progression of COVID-19. Such insights can identify potential targets for drug development and intervention strategies aimed at reducing morbidity and mortality.
The goal of this review was to review the different mechanisms by which TMAO produced by the gut microbiome is associated with the promotion of inflammation mechanisms, thrombosis, and expression of SR-B1 associated with COVID-19 infection development. We hypothesize that TMAO produced by the gut microbiome may increase the severity of COVID-19.
2. Evidence Acquisition
2.1. Metabolism of TMAO
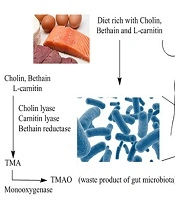
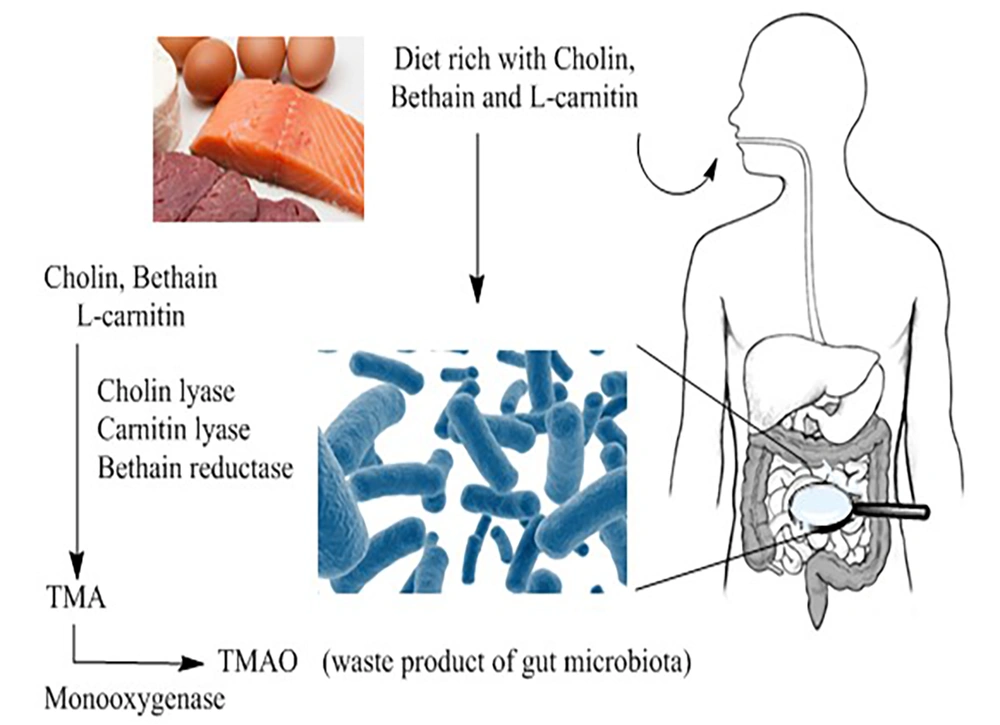
The exact biological metabolism of TMAO in humans remains poorly understood. Trimethylamine N-oxide is a waste product resulting from the oxidation of TMA, which is produced by the gut microbiome when it metabolizes choline-containing compounds found in the diet, such as choline, L-carnitine, and betaine (21). Various enzymes, including betaine reductase, choline TMA lyase, and carnitine TMA lyase, play a role in converting these choline-containing products into TMA within the intestinal lumen (22). Trimethylamine is then either oxidized by microorganisms within the gut through TMA monooxygenase or transported to the liver, where it undergoes oxidation by flavin monooxygenase (23) (Figure 1). These findings suggest a potential link between dietary and chemical metabolisms mediated by the gut microbiome, influencing various aspects of biological pathways.
2.2. The Association Between TMAO Secreted by Gut Microbiome and Inflammation
Several studies have indicated an association between TMAO dependent on gut microbiota and systemic inflammation (8): Gut-derived TMAO upregulates various molecular mechanisms associated with inflammation. Some studies have demonstrated that TMAO, originating from the gut microbiome, can enhance the expression of heat shock proteins (HSPs), which may contribute to the abnormal activation of macrophages involved in foam cell formation (24). Trimethylamine N-oxide can induce the expression of different proteins, such as HSP60 and GRP78, responsible for endoplasmic reticulum stress induction (25). Furthermore, TMAO stimulates the expression of SRs on the surface of macrophages, thereby contributing to the uptake of oxidized low-density lipoprotein (ox-LDL) and foam cell formation (26, 27). Scavenger receptors induce the expression of SR-A and CD36 on the surface of macrophages, which can identify ox-LDL molecules. Wei et al. have indicated that the inhibition of TMAO production through antibiotic therapy can reduce the number of macrophages and foam cell formation in mice (12). Moreover, several investigations have reported that TMAO increases CD36 expression and foam cell formation by inducing MAPK and c-Jun N-terminal kinase (JNK) signaling pathways (28, 29). Geng et al. have shown that using MAPK and JNK pathway inhibitors can reduce foam cell formation by decreasing CD36 expression (28).
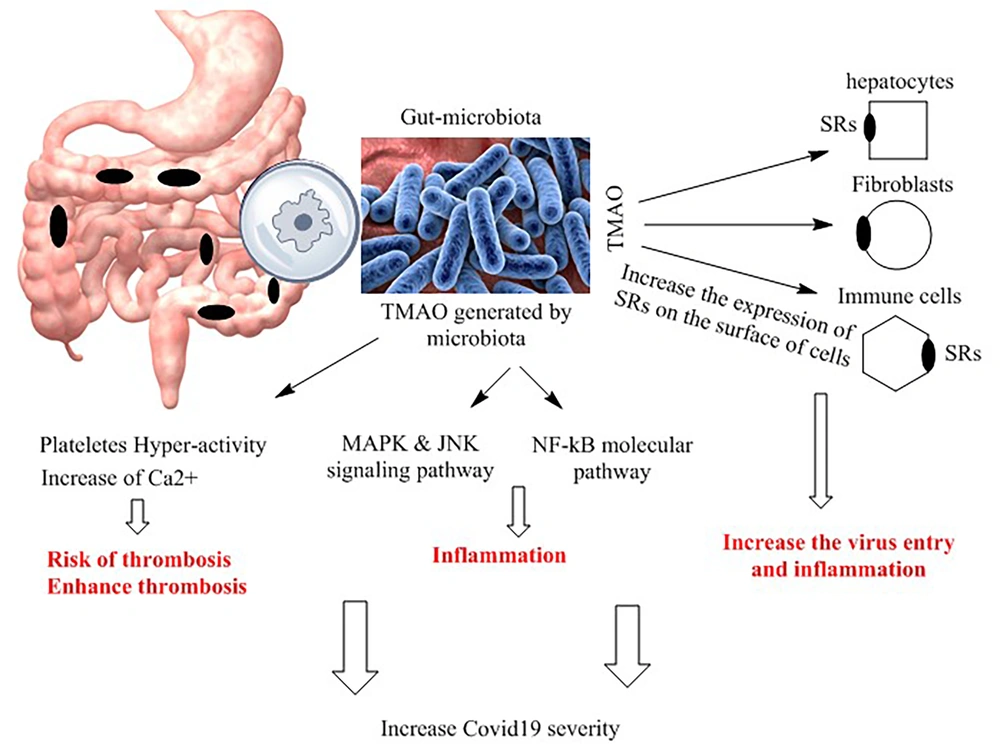
In addition, multiple studies have demonstrated that higher levels of TMAO increase the expression of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin 1beta (IL1β) while downregulating the expression of the anti-inflammatory cytokine IL-10 (8). Chuo et al. have shown a positive association between TMAO levels, IL-1β, and high-sensitivity C-reactive protein (hs-CRP) (30). In vitro studies conducted on cultured endothelial progenitor cells (EPCs) have reported that gut-derived TMAO induces cellular inflammation and oxidative stress (30). The nuclear factor-kappa B (NF-κB) pathway plays a crucial role in the expression of pro-inflammatory genes (31). Seldin et al. have demonstrated that increasing TMAO levels through a choline-rich diet in mice enhances the expression of inflammatory genes via an effect on the NF-κB pathway (32). They found that gut-derived TMAO increases several pro-inflammatory molecules, including E-selectin, interleukin 6 (IL-6), cyclooxygenase 2, and intracellular adhesion molecule 1 (ICAM1), by activating NF-κB (32). Ottinger et al. have reported that TMAO is related to poor outcomes in patients with acute inflammatory pneumonia. They measured the TMAO levels in blood samples from 317 patients with acute pneumonia and found a significant association between TMAO and mortality (33). This evidence suggests a link between TMAO produced by the gut microbiota and inflammation through various mechanisms, which could exacerbate the viral outcome of COVID-19 infection. The association between gut-derived TMAO and molecular mechanisms related to inflammation is summarized in Figure 2.
Mechanisms of trimethylamine N-oxide (TMAO) action. Trimethylamine N-oxide, through various molecular mechanisms, enhances inflammation, thrombosis, and virus entry into cells. It upregulates the expression of scavenger receptors (SRs) on the surfaces of different cells, facilitating virus entry. Furthermore, TMAO activates different molecular pathways, such as nuclear factor kappa (NF-kB), mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK), promoting inflammation and exacerbating COVID-19 severity.
2.3. Relationship Between TMAO Produced by the Gut Microbiome, SR-B1 Expression, and COVID-19
SRs-B1 are cell-surface HDL receptors that mediate the uptake or influx of HDL-derived cholesteryl-esters into cells and tissues (34). Previous studies have reported that SR-B1 is an essential receptor that affects HCV entry (35). However, there is not enough information about the potential roles of SR-B1 in SARS-CoV-19 infection. Few studies have demonstrated that SR-B1 receptors facilitate SARS-CoV-2 cell entry. Henrich et al. have shown that human cells expressing SR-B1 are susceptible to SARS-CoV-2 infection (36). Major cell types targeted by SARS-CoV-2, such as hepatocytes, immune cells, fibroblasts, adipocytes, and type II pneumocytes, express SR-B1 to contribute to viruses' entry and virus effects (37-39). In another study, Palacios-Rapalo et al. have shown that the SARS-CoV-2 S protein may bind to the cholesterol of HDL and enter the target cells. They have found that SR-B1 mediates SARS-CoV-2 attachment and transfer to the cells (40). On the other hand, some investigations have reported that TMAO produced by gut microbiota upregulates the expression of SRs (12). Animal studies demonstrated that dietary supplementation with TMAO increases cholesterol deposition into the peripheral tissues in mice (41). Chen et al. have indicated that TMAO induces the expression of genes related to cholesterol metabolism, such as SR-B1. However, this effect was TMAO dose-dependent (42). More information is needed to better estimate gut TMAO influences on SR-B1 expression (Figure 2).
2.4. Trimethylamine N-oxide Secreted by the Gut Microbiome Increases the Risk of Thrombosis
Disseminated intravascular coagulation and thrombosis are common complications in COVID-19 infections. COVID-19 induces a pro-thrombotic state, and the high incidence of reported major thrombotic events raises concerns about unique pro-thrombotic pathophysiology (43, 44). Autopsies of patients have revealed diffuse alveolar damage and microthrombi not only in pulmonary vasculature but also in other organs (45). Studies have reported that thrombotic events occur in up to one-third of COVID-19 patients, primarily as pulmonary emboli, and are associated with more severe disease and increased mortality (46).
Pyrin domain-containing 3 (NLRP3) interacts with apoptosis-associated speck-like protein (ASC) to cleave caspase-1, leading to the maturation and secretion of pro-inflammatory cytokines IL-18 and IL1b, which trigger inflammatory responses and play a crucial role in promoting the development of lipid plaques and destabilizing atherosclerotic plaques and thrombosis (47). The up-regulation of cellular adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1) and ICAM-1, plays an initial role in atheromatous plaque formation (48). This underscores the importance of effective thromboprophylaxis and the treatment of thrombotic complications in COVID-19 patients, especially those requiring intensive care.
Regarding the association between gut TMAO and the risk of thrombosis, Zhu et al. reported on the role of TMAO in platelet hyperreactivity (11). They found that in patients with an increased incidence of thrombotic events, TMAO concentrations were significantly elevated. Furthermore, in vitro studies have shown that TMAO increases platelet aggregation and adhesion to collagen. In the same experiment, the injection of TMAO in mice stimulated carotid thrombus formation with shortened occlusion time. Several animal studies have demonstrated the biological role of gut microbiota in thrombosis. Mice fed a choline-rich diet and supplemented with TMAO exhibited platelet aggregation and shortened occlusion time. Administration of antibiotics to mice receiving TMAO supplementation prevented platelet aggregation. In these findings, Zhu et al. reported that TMAO does not directly induce platelet activation but rather increases the release of Ca2+ and platelet activation in response to agonists (11).
In a human study, Zhu et al. demonstrated that dose-dependent choline supplementation increased platelet aggregation, which was correlated with TMAO levels. Aspirin treatment in combination with choline reduced both TMAO concentrations and platelet hyperactivity (49). The authors suggest that aspirin may alter gut microbiota composition, affecting its function and the production of metabolites. Altering gut microbiota with probiotic species has shown promise in the treatment of animal and human diseases. However, further research is needed to determine whether probiotic administration can specifically target TMAO produced by gut microbiomes.
Various studies have reported that probiotics can modulate innate and adaptive immune responses, regulate host-pathogen interactions, and stimulate the secretion of immunoglobulin A (50, 51). Several randomized clinical trials have demonstrated the potential benefits of probiotics in patient outcomes after surgery, reducing infectious and non-infectious complications (52, 53). Zeng's study reported the positive effects of probiotics in reducing ventilator-associated pneumonia in mechanically ventilated patients in the intensive care unit (54). The beneficial impact of probiotics on lung infections may be associated with the influence of gut microbiota on lung immunity, known as the gut-lung axis (55). Studies have indicated that microbiota can enhance resistance to viruses and pathogenic attacks on respiratory mucosa by improving the systemic immune response and preventing virus entry via the ACE2 receptor (56, 57).
To mitigate the production of TMAO by microorganisms, it is essential to block its synthesis pathway or reduce the population of responsible bacteria. This can be achieved through the use of broad-spectrum antibiotics or by introducing specific bacterial strains that limit or decrease the survival of other strains in the same niche, as in the case of probiotics.
3. Conclusions
Numerous studies have confirmed the significant role of the microbiota in synthesizing and releasing important metabolites with various biological properties, including TMAO. TMAO, produced by the gut microbiome, may potentially exacerbate the severity of COVID-19 through various mechanisms. This evidence underscores the importance of dysbiosis, which can lead to disease severity by producing different metabolites. The administration of prebiotics and probiotics to modulate the gut microbiome towards beneficial bacteria, thereby regulating pathways that generate proatherogenic metabolites, is proposed as a novel therapeutic strategy for COVID-19. However, additional studies, including animal models and clinical trials, are required to elucidate the underlying mechanisms and determine whether interventions targeting the gut microbiota and TMAO could help prevent atherosclerosis in COVID-19.