1. Background
Neonatal sepsis, caused by bacterial infection, remains a significant cause of mortality and morbidity in the neonatal population (1). Clinical sepsis occurs in the first 4 weeks of life, along with subsequent systemic symptoms, which are confirmed by a positive culture from sterile body fluids (proven sepsis) (2). In developed countries, sepsis affects 1 - 4 out of every 1 000 live births, with a higher prevalence in term boys compared to term girls. The occurrence of this gender preference is less common in preterm neonates (3).
Neonatal sepsis can present with subtle and varied symptoms, similar to other non-infectious diseases. These may include fever, hypothermia, poor feeding, lethargy, tachypnea, and tachycardia. Delayed diagnosis and treatment can have serious consequences, including severe morbidity and mortality (4).
Since blood culture results take 48 - 72 hours, other lab findings can aid in early neonatal sepsis diagnosis. The laboratory findings in neonatal sepsis and non-sepsis differ significantly. In neonatal sepsis, there is a decrease in the number of white blood cells (WBCs), specifically neutrophils, whereas in non-sepsis cases, the WBC count is typically within the normal range. Furthermore, neonatal sepsis is characterized by an increase in immature neutrophils referred to as band cells, whereas the number of band cells is usually low or absent in non-sepsis conditions. Other laboratory findings associated with neonatal sepsis include elevated levels of C-reactive protein (CRP), a marker of inflammation, and increased procalcitonin levels. On the other hand, non-sepsis cases may exhibit mild elevations in CRP and procalcitonin levels, but these are generally not as high as observed in neonatal sepsis (5).
The red cell distribution width (RDW) test examines variations in red blood cell volume and size and can help in diagnosing neonatal sepsis early (3). The sepsis cascade, characterized by inflammation and oxidative stress, has been shown to have effects on red blood cell (RBC) survival and maturation. These effects can lead to the release of premature RBCs into circulation and increase the levels of RDW. It is especially useful when paired with other clinical symptoms and lab findings (6).
Platelets undergo a similar process. Increased platelet production in the bone marrow can explain the high levels of mean platelet volume (MPV) and platelet distribution width (PDW) in septic patients (7). Therefore, platelet indices, platelet count, PDW, and MPV may play a significant role in diagnosing neonatal sepsis (8). Early sepsis diagnosis could potentially benefit from considering additional blood indices, such as the count of monocytes and eosinophils (9).
Currently, blood culture results are considered the gold standard for diagnosing sepsis. However, these results typically have a delay of at least 48 to 72 hours, and only 30 to 70% of septic cases show a positive outcome (10). Given this time delay, it is crucial to explore laboratory diagnostic methods that are both reliable and faster, as they can enable timely initiation of treatment. Researchers aim to develop an early diagnostic method for neonatal sepsis to avoid unnecessary antibiotic treatment and the subsequent development of drug resistance in high-risk newborns (10, 11). Numerous studies have been conducted on the early diagnosis of neonatal sepsis during the last two decades (12).
2. Objectives
We examined the relationship between neonatal sepsis and blood indices in order to achieve early diagnosis.
3. Methods
3.1. Study Population
The present study was a hospital-based retrospective multiple-event case-control study. The study cases included all newborns admitted with the diagnosis of sepsis, including early and late onset (EOS and LOS), to the neonatal ward and NICU of the Children's Medical Center and Bahrami Hospital between 2013 and 2022. Our control group included neonates hospitalized with laboratory sepsis without a positive culture, as well as neonates who were admitted for reasons other than infection, such as hyperbilirubinemia (not severe cases). Matching was done in the control group in terms of demographic characteristics.
3.2. Study Design
The study population was divided into three groups. The initial group consisted of newborns with confirmed sepsis and a positive blood culture. The second group consisted of neonates with culture-negative sepsis (laboratory sepsis). In laboratory sepsis, the laboratory data criteria include leukocyte count < 5000 or ≥ 20000/mm3, platelet < 150000, absolute neutrophil count (< 1500 or ≥ 5000/mm3), CRP level > 6 mg/dl, and immature/total neutrophil count ≥ 0.2 (5). Furthermore, the third group comprised neonates without sepsis who were admitted to the wards for other medical conditions. The demographic and laboratory data of the three groups were then compared. Inclusion criteria comprised neonates who have positive blood cultures or neonates with at least two laboratory diagnoses of sepsis (5). Also, several non-septic infants were included in the study as a control group, such as hyperbilirubinemia (not severe cases).
3.3. Exclusion Criteria
- Infants with positive blood cultures admitted to other wards
- Infants with symptoms of sepsis but lack any positive blood cultures in the case group or any laboratory criteria for sepsis in the control group
- Staphylococcus epidermidis-positive blood cultures due to contamination, especially if the patient is asymptomatic
- Patients with severe hyperbilirubinemia in the control group (13)
- Patients with missing information in their medical files
3.4. Data Collection
The questionnaire included the date of admission, age, weight, gender of the neonate, gestational age, kind of microorganism, number of leukocytes, eosinophil, neutrophils, RDW, MPV, PDW, CRP, and platelet counts. A pediatric resident filled out the questionnaire based on medical records, the hospital HIS system, and nursing report sheets. BACTEC laboratory equipment was used to check positive blood cultures. To evaluate blood indices, we checked blood cells with a CBC sample using a colter counter. CRP was checked by a quantitative test.
3.5. Statistical Analysis
We analyzed the collected data using descriptive statistical tests, chi-square test, Fisher’s test, and paired t-test, and to compare the means of three groups, we used one-way ANOVA with SPSS version 22 software. The normality of the data distribution was determined using the Kolmogorov-Smirnov test. A P-value < 0.05 was considered significant.
3.6. Ethics
This study was approved by the Ethics Committee of Tehran University of Medical Sciences with Ethics Code IR.TUMS.CHMC.REC.1399.147.
4. Results
The study population consisted of 319 neonates. Among them, 209 cases were diagnosed with culture-positive sepsis, 65 cases had culture-negative sepsis, and 45 cases did not have neonatal sepsis. The mean birth gestational age was 37.5 ± 1.25 weeks, and the mean birth weight was 2999 ± 445 g. Although the proportion of positive cultures in pre-term babies (60.9%) was slightly higher than in full-term babies (54.4%), this difference was not statistically significant (P = 0.288). In terms of gender, culture-positive sepsis was more common in boys (57%) than in girls (43%). However, this difference was also not statistically significant (P = 0.918). LOS and nosocomial infections were more common than EOS in neonates with positive cultures in our study, conducted in two referral hospitals, with patient numbers of 96, 64, and 49, respectively.
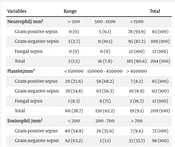
According to Table 1, Pearson's correlation coefficient showed that thrombocytopenia, positive CRP, and high RDW were correlated with culture-positive sepsis (P = 0.000), indicating a significant statistical difference between the three groups. It was also found that there was no significant relationship between the three groups (culture-positive sepsis, culture-negative sepsis, and no-sepsis) in terms of neutrophil count (P = 0.10), eosinophil count (P = 0.757), PDW (P = 0.234), and MPV (P = 0.524). Although no significant relationship was found between the neutrophil count and infection (Pearson's coefficient = 0.10), the data showed that 60% of neonates with a neutrophil count less than 500 mm3 had culture-positive sepsis, compared to 40% with negative cultures. However, this difference was not statistically significant. The frequency of studied variables among types of microorganisms in blood culture-positive neonates is also shown in Table 2. The present study found no significant relationship between the type of microorganism (including Gram-negative and positive bacteria and fungi) and the neutrophil count (P = 0.251).
| Variables | Groups | Total | Pearson’s Chi-squared | ||
|---|---|---|---|---|---|
| Culture-Positive | Culture-Negative | Non-sepsis | |||
| Neutrophil countaccording to neutrophil count, mm3 | 0.10 | ||||
| < 500 | 3 (60) | 2 (40) | 0.0 (0.0) | 5 (100) | |
| 500 - 1500 | 16 (72.7) | 2 (9.1) | 4 (18.2) | 22 (100) | |
| < 1500 | 185 (64.7) | 61 (21.3) | 40 (14) | 286 (100) | |
| Total | 204 (65.1) | 65 (20.8) | 44 (14.1) | 313 (100) | |
| Platelet count according to platelet count, mm3 | 0.000 | ||||
| < 150000 | 60 (92.3) | 5 (7.7) | 0.0 (0.0) | 65 (100) | |
| 150000 - 450000 | 130 (57.8) | 53 (23.5) | 42 (18.7) | 225 (100) | |
| > 450000 | 19 (65.6) | 7 (24.1) | 3 (10.3) | 29 (100) | |
| Total | 209 (65.6) | 65 (20.3) | 45 (14.1) | 319 (100) | |
| Platelet countaccording to eosinophil count, mm3 | 0.757 | ||||
| < 200 | 106 (67.6) | 34 (21.6) | 17 (10.8) | 157 (100) | |
| 200 - 700 | 62 (56.4) | 27 (24.5) | 21 (19.1) | 110 (100) | |
| > 700 | 14 (56) | 4 (16) | 7 (28) | 25 (100) | |
| Total | 182 (62.3) | 65 (22.3) | 45 (15.4) | 292 (100) | |
| Positive | 78 (74.3) | 22 (20.9) | 5 (4.8) | 105 (100) | 0.000 |
| Negative | 127 (64.8) | 42 (21.4) | 27 (23.8) | 196 (100) | |
| Total | 205 (68.1) | 64 (21.3) | 32 (10.6) | 301 (100) | |
| Platelet count according to RDW amount, mm3 | 0.000 | ||||
| > 16.5 | 135 (77.6) | 21 (12.1) | 18 (10.3) | 174 (100) | |
| ≤ 16.5 | 50 (41.3) | 44 (36.4) | 27 (22.3) | 121 (100) | |
| Total | 185 (62.7) | 65 (22.1) | 45 (15.2) | 295 (100) | |
| Platelet count according to PDW, mm3 | 0.234 | ||||
| < 17.5 | 16 (66.7) | 7 (29.2) | 1 (4.1) | 24 (100) | |
| ≤ 17.5 | 174 (63) | 58 (21.1) | 44 (15.9) | 276 (100) | |
| Total | 190 (63.3) | 65 (21.7) | 45 (15) | 300 (100) | |
| Platelet count according to MPV, mm3 | 0.524 | ||||
| > 10 | 140 (62.8) | 49 (22) | 34 (15.2) | 223 (100) | |
| < 10 | 53 (66.2) | 16 (20) | 11 (13.8) | 80 (100) | |
| Total | 193 (63.7) | 65 (21.5) | 45 (14.8) | 303 (100) | |
Abbreviations: MPV, mean platelet volume; PDW, platelet distribution width; RDW, red cell distribution width.
| Variables | Range | Total | ||
|---|---|---|---|---|
| Neutrophil/mm3 | > 500 | 500 - 1500 | < 1500 | |
| Gram-positive sepsis | 0 (0) | 5 (6.1) | 78 (93.9) | 83 (100) |
| Gram-negative sepsis | 3 (2.7) | 11 (10.1) | 95 (87.2) | 109 (100) |
| Fungal sepsis | 0 (0) | 0 (0) | 12 (100) | 12 (100) |
| Total | 3 (1.5) | 16 (7.9) | 185 (90.6) | 204 (100) |
| Platelet/mm3 | < 150000 | 150000 - 450000 | > 450000 | |
| Gram-positive sepsis | 20 (23.6) | 58 (68.2) | 7 (8.2) | 85 (100) |
| Gram-negative sepsis | 39 (34.8) | 63 (56.3) | 10 (8.9) | 112 (100) |
| Fungal sepsis | 1 (8.3) | 9 (75) | 2 (16.7) | 12 (100) |
| Total | 60 (28.7) | 130 (62.2) | 19 (9.1) | 209 (100) |
| Eosinophil/mm3 | < 200 | 200 - 700 | > 700 | |
| Gram-positive sepsis | 40 (54.8) | 26 (35.6) | 7 (9.6) | 73 (100) |
| Gram-negative sepsis | 62 (63.2) | 3 (3.1) | 33 (33.7) | 98 (100) |
| Fungal sepsis | 4 (36.4) | 4 (36.4) | 3 (27.2) | 11 (100) |
| Total | 106 (58.2) | 33 (18.1) | 43 (23.6) | 182 (100) |
| CRP, mg/dL | > 6 | ≥ 6 | ||
| Gram-positive sepsis | 48 (57.8.) | 35 (42.2) | 83 (100) | |
| Gram-negative sepsis | 70 (62.5) | 42 (37.5) | 112 (100) | |
| Fungal sepsis | 9 (90) | 1 (10) | 10 (100) | |
| Total | 127 (61.9) | 78 (38.1) | 205 (100) | |
| RDW, fL | < 16.5 | ≤ 16.5 | ||
| Gram-positive sepsis | 54 (72) | 21 (28) | 75 (100) | |
| Gram-negative sepsis | 74 (74.7) | 25 (25.3) | 99 (100) | |
| Fungal sepsis | 7 (63.5) | 4 (36.5) | 11 (100) | |
| Total | 135 (73) | 50 (27) | 185 (100) | |
| PDW, fL | < 17.5 | ≥ 17.5 | ||
| Gram-positive sepsis | 2 (2.5) | 77 (97.5) | 79 (100) | |
| Gram-negative sepsis | 13 (13.1) | 86 (86.9) | 99 (100) | |
| Fungal sepsis | 1 (8.3) | 11 (91.7) | 12 (100) | |
| Total | 16 (8.5) | 174 (91.5) | 190 (100) | |
| MPV, fL | ≤ 11 | < 10 | ||
| Gram-positive sepsis | 59 (74.6) | 20 (25.4) | 79 (100) | |
| Gram-negative sepsis | 72 (70.5) | 30 (29.5) | 102 (100) | |
| Fungal sepsis | 9 (75) | 3 (25) | 12 (100) | |
| Total | 140 (72.5) | 53 (27.5) | 193 (100) | |
Abbreviations: MPV, mean platelet volume; PDW, platelet distribution width; RDW, cell distribution width; CRP, C-reactive protein.
Additionally, there was no significant relationship between the eosinophil count and sepsis, as indicated by Pearson's coefficient of 0.757. Furthermore, the comparison of studied variables between three groups (Gram-positive culture, Gram-negative culture, and fungal culture) showed no significant relationship between the type of microorganism and eosinophil count (P = 0.075) (Table 3). However, it is worth mentioning that the mean eosinophil count was significantly higher in infants with positive fungal culture (mean: 803.64) compared to those with gram-negative culture (mean: 216.14) (P = 0.025) (Tables 4 and 5).
| Variables | White Blood Cell | Neutrophil | Platete | Eosinophil | C-Reactive Protein | Red Cell Distribution Width | Platelet Distribution Width |
|---|---|---|---|---|---|---|---|
| Chi-square | 1.469 | 2.768 | 4.441 | 5.177 | 8.788 | 2.295 | 1.303 |
| Df | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Asymp. Sig. | 0.480 | 0.251 | 0.109 | 0.075 | 0.012 | 0.317 | 0.521 |
a P-values obtained from the Kruskal-Wallis test.
b Grouping variable: Germ group.
| Germ Group | White Blood Cell | Neutrophil | Platete | Eosinophil | CRP | RDW | PDW | MPV |
|---|---|---|---|---|---|---|---|---|
| Gram-positive | ||||||||
| Valid | 85 | 83 | 85 | 73 | 83 | 75 | 79 | 79 |
| Missing | 0 | 2 | 0 | 12 | 2 | 10 | 6 | 6 |
| Mean | 11721.29 | 6324.75 | 271576.47 | 979.84 | 23.30 | 26.8253 | 14.047 | 9.33 |
| Gram-negative | ||||||||
| Valid | 112 | 109 | 112 | 98 | 112 | 99 | 99 | 102 |
| Missing | 0 | 3 | 0 | 14 | 0 | 13 | 13 | 10 |
| Mean | 11118.04 | 6079.93 | 225583.04 | 216.14 | 32.04 | 30.6676 | 14.330 | 9.47 |
| Fungal | ||||||||
| Valid | 12 | 12 | 12 | 11 | 10 | 11 | 12 | 12 |
| Missing | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 |
| Mean | 11859.17 | 5934.25 | 275083.33 | 803.64 | 51.50 | 40.9182 | 15.083 | 9.71 |
Abbreviations: MPV, mean platelet volume; PDW, platelet distribution width; RDW, cell distribution width; CRP, C-reactive protein.
| Variables | White Blood Cell | Neutrophil | Platete | Eosinophil | CRP | RDW | PDW |
|---|---|---|---|---|---|---|---|
| Mann-Whitney U test | 537.000 | 544.000 | 542.500 | 317.000 | 334.500 | 471.000 | 516.000 |
| Wilcoxon test | 6865.000 | 6539.000 | 6870.500 | 5168.000 | 6662.500 | 5421.000 | 5466.000 |
| Z-score | -1.141 | -0.954 | -1.095 | -2.234 | -2.107 | -0.732 | -0.741 |
| Asymp. Sig. (2-tailed) | 0.254 | 0.340 | 0.274 | 0.025 | 0.035 | 0.464 | 0.459 |
a Grouping variable: Germ group.
The normality of the data was checked using the Kolmogorov-Smirnov test. Except for MPV (P = 0.24) in the ANOVA, the mean values of the data were not significantly different between the groups (P = 0.42). However, the other data had a non-normal distribution. Therefore, non-parametric tests were used to compare the means.
In the culture-positive group, when comparing the three groups of Gram-positive culture, Gram-negative culture, and fungal culture, only CRP showed a significant difference (P = 0.012) (Table 3). The mean CRP levels were 51.5 in the fungal culture group, 32.04 in the Gram-negative culture group, and 23.3 in the Gram-positive culture group (Table 4).
5. Discussion
Effective and timely use of antibiotics is crucial in the treatment of neonatal sepsis, a potentially life-threatening condition. However, unnecessary use of antibiotics before a definitive diagnosis can contribute to the development of drug resistance. It is crucial for healthcare providers to balance the urgency of treatment with the judicious use of antibiotics, ensuring proper diagnostic evaluation before starting therapy.
In this hospital-based retrospective multiple-event case-control study, we compared demographic and lab data in three groups of culture-positive sepsis, culture-negative sepsis, and neonates without sepsis to provide useful information for the early diagnosis of neonatal sepsis.
In this study, although the proportion of positive cultures was slightly higher in pre-term and male babies compared to full-term and female babies, consistent with the findings of other studies (14, 15), this difference was not statistically significant. In our study, we did not find a significant relationship between neutrophil count and infection. Additionally, we did not observe a significant relationship between the type of microorganism and the neutrophil count. However, in a study conducted by Can et al. in 2018, they observed a total of 122 neonates, 78 of whom had EOS. Their findings showed that a neutrophil-to-lymphocyte ratio (NLR) of 6.76 was determined as the predictive cut-off value for EOS, with a sensitivity of 94.7% and specificity of 100%. It is important to note that our study was conducted in two referral hospitals, resulting in a lower incidence of EOS among our patients compared to the study conducted by Can et al. (8).
In the current study, we observed a correlation between thrombocytopenia and culture-positive sepsis. Previous studies on predictive laboratory indices for sepsis have also demonstrated a relationship between thrombocytopenia and neonatal sepsis (16, 17). Our study showed no significant relationship between the type of microorganism (Gram-positive bacteria, Gram-negative bacteria, and fungus) and the number of platelets (P = 0.109). These results are consistent with the findings of another study in this field (18).
The present study found no significant relationship between eosinophil count and sepsis (Pearson’s coefficient = 0.757). Additionally, there was no significant relationship between the type of microorganism and eosinophil count (P = 0.075). However, the mean eosinophil count was significantly higher in infants with a positive fungal culture compared to those with a Gram-negative culture. It is important to note that previous research on the correlation between eosinopenia and sepsis has produced inconsistent results. For example, Wilar observed that the mean eosinophil count was significantly lower in cases of EOS (P < 0.001). The diagnostic value of eosinopenia in their sepsis group (cutoff point: 140 cells/mm3) showed a sensitivity and specificity of 60% and 90.0%, respectively (19). Lee also reviewed and mentioned eosinopenia as an old biomarker for infection, which may be useful in differentiating sepsis from non-infectious conditions. They reported that eosinopenia of less than 50 cells/mm3 has sensitivity, specificity, and positive and negative predictive values of 81%, 65%, 66%, and 80%, respectively, for adult sepsis diagnosis (9).
In the current study, 74.3% of infants with a positive CRP had positive-culture sepsis, which was found to be statistically significant (P = 0.00). Omar and Mohammad also reported a significant relationship between culture-positive sepsis, CRP, and increased RDW (P-value 0.01) (20). Furthermore, Ellahony et al. found a strong positive correlation between RDW and CRP (r = 0.8; P < 0.0001) (6). CRP has been identified as an important predictive parameter in other studies, demonstrating a strong relationship with sepsis diagnosis (14, 21). However, it is worth noting that in our study, 65% of infants with a negative CRP still had positive-culture sepsis. It is important to highlight that we only recorded the initial CRP at the time of culture-positive and did not include serial CRP measurements. If we had considered serial CRP measurements, there might have been a higher proportion of positive CRP results, as Chaudhuri et al. discussed the role of serial CRP in the diagnosis of neonatal sepsis (22). In addition, we found a significant difference in CRP levels (P = 0.012) between cases of Gram-positive, Gram-negative, and fungal sepsis. Interestingly, the mean CRP levels were higher in the fungal culture group compared to both the Gram-negative and Gram-positive culture groups. Therefore, based on these findings, we suggest considering empirical antifungal treatment in cases with elevated CRP levels, particularly when accompanied by eosinophilia.
In the present study, 77.6% of infants with an RDW above 16.5 had culture-positive sepsis, which was found to be statistically significant (P = 0.00). Deka and P. conducted a prospective observational study involving 50 normal and 50 septic neonates. They found that the mean RDW was higher in septic neonates (18.59 ± 1.28) compared to normal newborns (16.21 ± 1.35). In the sepsis group, RDW was found to have a statistically significant correlation with CRP. They also identified an RDW cut-off level of 17.25%, which had 86% sensitivity, 87% specificity, and 93.5% accuracy in diagnosing neonatal sepsis (3). Another study by Omer and Mohammed also considered RDW as a simple method for diagnosing neonatal sepsis (20). Additionally, some studies suggest that RDW may be the most useful predictor of mortality and a strong indicator of sepsis severity (23).
No significant difference was found between PDW and sepsis in the current study (P = 0.234). There was also no relationship between the type of microorganism and PDW, nor between the type of microorganism and MPV. However, a meta-analysis conducted by Wang et al., involving 932 neonates, demonstrated that MPV levels were higher in patients with neonatal sepsis compared to healthy controls. As a result, Wang et al. concluded that MPV could potentially serve as an indicator for the early diagnosis of neonatal sepsis (24).
In conclusion, our retrospective case-control study found that neonates with culture-positive sepsis had a significant correlation with thrombocytopenia, positive CRP, and high RDW. These findings suggest that these lab parameters could be potential indicators of culture-positive sepsis in neonates. Additionally, within the culture-positive group, neonates with fungal sepsis had higher mean CRP and eosinophil levels. These results highlight the importance of monitoring these lab parameters in neonates suspected of sepsis, especially in the presence of fungal infection. Further prospective studies are warranted to validate our findings and explore potential mechanisms underlying these associations.
It is important to acknowledge the limitations of our study, specifically EOS, due to the inclusion of referral hospitals in our study sample. Additionally, we observed a limited number of cases with fungal-positive cultures, which hindered our ability to draw definitive conclusions regarding the association between fungi and sepsis, particularly concerning the absence of serial CRP measurements. Incorporating such serial measurements could offer more precise and detailed information on the connection between CRP, EOS, and sepsis.