1. Background
Based on 2021 data, every day, 4 000 people worldwide become infected with HIV, and every minute, a person dies from AIDS-related causes, resulting in 650 000 deaths annually (1). Eastern Europe and Central Asia are experiencing the fastest-growing HIV epidemic in the world (2). In 2021, 160,000 people were newly infected with HIV in Eastern Europe, with the highest rates recorded in the Russian Federation at 40.2 per 100,000 population. Additionally, 13% of newly diagnosed patients in the Russian Federation had CD4+ lymphocyte counts of less than 200/mm3 (2).
According to the Russian Federal Register of COVID-19 survivors, for the study period from March 1, 2020, to December 31, 2022, a total of 21,798,509 patients with COVID-19 were identified in the Russian Federation. Among them, 393 712 patients died. Additionally, there were 24,444 patients with COVID-19 and HIV coinfection, of which 4960 patients died (3).
2. Objectives
3. Methods
During the study period, a total of 3,563 patients were hospitalized at Infectious Diseases Hospital No. 2 in Moscow due to COVID-19. Out of these, 2,962 patients did not meet the inclusion criteria for the study.
Inclusion criteria were a positive PCR test result for SARS-CoV-2 in a sample of nasopharyngeal and oropharyngeal swabs, radiological infiltrates of COVID-19 on chest CT scans, and an established diagnosis of advanced HIV disease according to the WHO definition, which includes a CD4+ lymphocyte count below 200 cells/mm3 or stage 3 or 4 according to the WHO clinical classification in adults and adolescents (7).
A total of 101 patients were excluded from the study due to various exclusion criteria: 38 patients had a tuberculosis diagnosis, 14 patients had severe liver failure, 28 patients had severe renal failure, 17 patients had other serious conditions that could potentially bias the study results, and 4 patients had insufficient time on hospitalization with insufficient data for analysis.
As a result, a total of 408 patients were included in the study. Among these 408 patients, 132 were newly diagnosed with HIV infection, while 276 patients had previously diagnosed advanced HIV disease (Figure 1).
No ethics committee approval was sought due to the retrospective design of the study. Our study was performed in accordance with the ethical standards of the Helsinki Declaration, which was accepted by the World Health Community in 1975 (revised in 2013).
The initial data were collected, adjusted, and systematized using Microsoft Office Excel 2016 spreadsheets. Statistical Package for the Social Sciences (SPSS) version 22 was used for statistical analysis. The significance of distribution function differences was determined using the non-parametric Mann-Whitney and Kolmogorov-Smirnov tests. The applicability of parametric statistical methods was assessed through calculations of skewness and kurtosis coefficients. Statistical significance was defined as P < 0.05.
4. Results
The primary outcome of our study was death.
We stratified all patients according to the severity of COVID-19 using the eight-step scale proposed by Beigel et al. (8). The characteristics of patients at the time of inclusion in the study are presented in Table 1.
| Index | Group 1 ( N = 132) | Group 2 ( N = 276) | P-Value b |
|---|---|---|---|
| Age; median (min - max) (y) | 43 (23 - 76) | 43 (21 - 71) | 0.207.0.607 |
| Gender (female) | 46.132 (34.8) | 93.276 (33.7) | 0.451 |
| Vaccination against COVID-19 | 24.138 (17.4) | 33.359 (9.2) | |
| Day from onset of COVID-19 symptoms at the time of hospitalization; median (min - max) | 16 (1 - 171) | 9 (1 - 130) | 0.017 |
| Score On Ordinal Scalec | |||
| Hospitalized, not requiring supplemental oxygen, requiring ongoing medical care (degree of physical impairment = 4) | 49.132 (37.1) | 165.276 (59.8) | 0.001 |
| Hospitalized, requiring supplemental oxygen (degree of physical impairment = 5) | 83.132 (62.9) | 111.276 (40.2) | < 0.001 |
| Hospitalized, receiving noninvasive ventilation or high-flow oxygen devices (degree of physical impairment = 6) | 30.132 (22.7) | 24.276 (8.4) | < 0.001 |
| Hospitalized, receiving invasive mechanical ventilation or ECMO (degree of physical impairment = 7) | 11.132 (8.3) | 12.276 (4.3) | < 0.001 |
| Admission to non-HIV medical center | 108.132 (81.8) | 77.276 (27.9) | < 0.001 |
| Prescription of immunobiological monoclonal antibodies | 37.126 (29.4) | 22.268 (8.2) | < 0.001 |
| Intensive care unit admission | 28.132 (21.2) | 25.275 (9.1) | 0.001 |
| Oxygen support | 102.132 (77.3) | 164.276 (59.4) | < 0.001 |
| Low flow oxygenation | 83.132 (62.9) | 111.276 (40.2) | < 0.001 |
| High flow oxygenation | 30.132 (22.7) | 24.276 (8.4) | < 0.001 |
| Mechanical ventilation | 11.132 (8.3) | 12.276 (4.3) | 0.083 |
| Proportion of Lung Tissue Damage According to the Results of Computed Tomography of the Lungs | |||
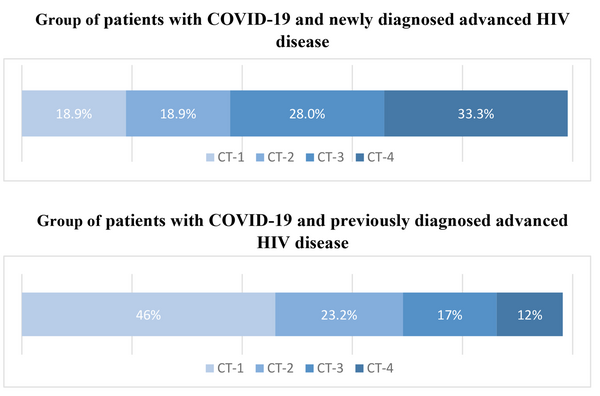
| CT 1 (up to 25%) | 25.131 (18.9) | 127.272 (46.0) | < 0.001 |
| CT 2 (up to 50%) | 24.131 (18.9) | 65.272 (23.2) | 0.127 |
| CT 3 (up to 75%) | 38.131 (28) | 46.272 (17) | 0.004 |
| CT 4 (more than 75%) | 44.131 (33.3) | 33.272 (12) | < 0.001 |
| Laboratory tests | |||
| Lymphocytes (absolute count); less than 1.0 × 109 | 87.129 (65.9) | 152.269 (55.1) | 0.024 |
| C-reactive protein; above 10 mg/L | 71.128 (55.5) | 206.266 (77.4) | < 0.001 |
| Platelets (absolute count); less than 150 × 109 | 29.130 (22.3) | 97.269 (36.1) | 0.004 |
| Ferritin; above 600 ng/L | 64.84 (76.2) | 98.143 (68.5) | 0.140 |
| Lactate dehydrogenase; above 460 U/L | 77.94 (81.9) | 137.185 (74.1) | 0.092 |
| D-dimer; above 250 ng/mL | 73.111 (65.8) | 168.225 (74.7) | 0.058 |
| Fibrinogen; above 3.97 g/L | 58.124 (46.9) | 160.258 (62) | 0.003 |
| Immune Status Indicators | |||
| CD 4+ > 500 cells/µL | 2.123 (1.6) | 4.267 (1.5) | 0.614 |
| CD 4+ 350 - 500 cells/µL | 2.123 (1.6) | 6.267 (2.2) | 0.512 |
| CD 4+ 200 - 349 cells/µL | 1.123 (0.8) | 18.267 (6.9) | 0.006 |
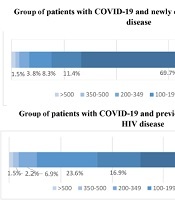
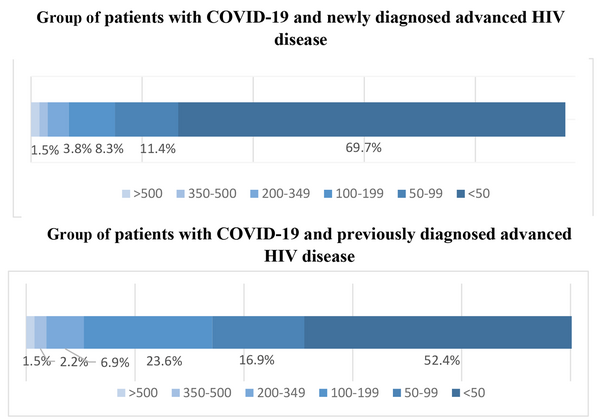
| CD 4+ 100 - 199 cells/µL | 11.123 (8.9) | 63.267 (23.6) | < 0.001 |
| CD 4+ 50 - 99 cells/µL | 15.123 (12.2) | 45.267 (16.9) | 0.150 |
| CD 4+ < 50 cells/µL | 92.123 (74.8) | 140.267 (52.4) | < 0.001 |
| Immunoregulatory index within reference values ( 1.20 - 2.50) | 1.123 (0.8) | 7.267 (2.6) | 0.223 |
| HIV viral load less than detectable level | 0.88 (0) | 29.154 (10.5) | < 0.001 |
| Opportunistic Infections and Other Conditions | |||
| Toxoplasmosis | 6.130 (4.6) | 17.276 (6.2) | 0.354 |
| Pneumocystis pneumoniad | 81.130 (62.3) | 85.276 (30.8) | < 0.001 |
| CMV pneumonitise | 66.130 (50.8) | 55.275 (20.0) | < 0.001 |
| Oropharyngeal candidiasis | 79.130 (60.8) | 142.275 (51.6) | 0.053 |
| Candidiasis of the gastrointestinal tract, respiratory organs, genitourinary system | 18.130 (13.8) | 28.275 (10.2) | 0.179 |
| Kaposi's sarcoma | 2.130 (1.5) | 4.275 (1.5) | 0.626 |
| Shingles | 4.129 (3.1) | 3.268 (1.1) | 0.159 |
| Encephalitis | 14.130 (10.8) | 35.273 (12.8) | 0.340 |
| Cryptococcosis | 2.129 (1.6) | 1.272 (0.4) | 0.243 |
| Oncological disease | 2.129 (1.6) | 6.276 (2.2) | 0.492 |
| Lymphoproliferative disease | 2.129 (1.6) | 4.273 (1.5) | 0.625 |
| Cancer of the vulva | 0.45 (0) | 1.91 (1.1) | 0.669 |
| Kidney cancer | 0.132 (0) | 1.276 (0.4) | 0.676 |
| Body mass index below 10% | 70.130 (53.8) | 112.275 (40.7) | 0.009 |
| Sepsis | 9.130 (6.9) | 18.273 (6.6) | 0.527 |
| Bacterial pneumonia | 59.130 (45.4) | 107.276 (38.8) | 0.124 |
| Fungal pneumonia | 29.130 (22.3) | 53.276 (19.2) | 0.274 |
| Urinary tract infection | 9.130 (6.9) | 23.276 (8.3) | 0.392 |
| Associated Chronic Infectious Agents | |||
| Chronic viral hepatitis C | 27.130 (20.8) | 125.275 (45.5) | < 0.001 |
| Chronic viral hepatitis C treated | 0.130 (0) | 4.276 (1.4) | 0.212 |
| Chronic viral hepatitis B | 6.130 (4.6) | 16.276 (5.8) | 0.409 |
| Traditional risk factors for severe COVID-19 | |||
| Diabetes | 1.130 (0.8) | 4.275 (1.5) | 0.485 |
| Obesity | 1.128 (0.8) | 5.276 (1.8) | 0.383 |
| Arterial hypertension | 10.130 (7.7) | 14.275 (5.1) | 0.204 |
| Chronic kidney disease | 0.130 (0) | 7.275 (2.5) | 0.065 |
| Gout | 0.128 (0) | 2.276 (0.7) | 0.466 |
Baseline Patient Characteristics a
The mortality rate in the group of patients with COVID-19 and advanced HIV disease (31.6% CI, 27.4 - 36.1%). The mortality rate in the group of patients with COVID-19 and newly diagnosed advanced HIV disease at the time of hospitalization (group 1) was 46.2% (CI, 38.2 - 54.3%), and in the group of patients with COVID-19 and a previously established diagnosis of advanced HIV disease (group 2), it was -24.6% (CI, 20.0 - 29.8%).
The study groups were comparable by gender (P = 0.451) and age (P = 0.67). Patients from group 1 were admitted to the specialized infectious hospital later than those from group 2 (median 16 and 8 days; P < 0.001) and stayed longer (Me 22 and 14 days; P < 0.001).
A total of 108 patients (81.8%) with COVID-19 and newly diagnosed advanced HIV disease were hospitalized in a non-specialized infectious center and were subsequently transferred to an infectious hospital No.2 in Moscow.
Patients from group 1 were significantly more likely to require oxygen support: 102/132 (77.3%) patients from group 1 compared to 164/276 (59.4%) patients from group 2 (P < 0.001). Patients in group 1 demonstrated more severe respiratory failure, requiring high-flow oxygen in 30/132 (22.7%) patients in group 1 compared to 24/276 (8.4%) patients in group 2 (P < 0.001).
However, 77 patients (27.9%) in group 2 kept silent about their HIV status, and they were hospitalized in other medical institutions.
Of these 77 patients who did not choose to disclose their HIV status in the outpatient setting, death occurred in 25 patients (32.5% CI, 23.4 - 42.7%). Fourteen patients (18.2%) were adherent to antiretroviral therapy (ART); the HIV viral load was below detectable levels only in 4 patients (5.2%; Table 2).
| Index | Patients with COVID-19 and Previously Known Advanced HIV Disease Who Did Not Choose to Disclose Their HIV Status ( N = 77) |
|---|---|
| Day of COVID-19 at the time of hospitalization median (min-max) | 13 (1 - 130) |
| Vaccination against COVID-19 | 9.75 (11.7) |
| Years passed since the diagnosis of HIV infection, median (min-max) | |
| Adherent to ART | 14.77 (18.2) |
| Suppressed HIV viral load | 4.48 (5.2) |
| CD 4+ > 500, cells/µL | 0.73 (0) |
| CD 4+ 350 - 500, cells/µL | 0.73 (0) |
| CD 4+ 200 - 349, cells/µL | 1.73 (1.3) |
| CD 4+ 100 - 199, cells/µL | 16.73 (21.9) |
| CD 4+ 50 - 99, cells/µL | 9.73 (12.3) |
| CD 4+ < 50, cells/µL | 47.73 (61) |
| Immunoregulatory index within reference values (1.20 - 2.50) | 1.73 (1.3) |
| Opportunistic infections and conditions | |
| Toxoplasmosis | 3.77 (3.9) |
| Pneumocystis pneumonia | 30.77 (39) |
| Manifest CMV infection with lung involvement | 22.77(28.6) |
| Oropharyngeal candidiasis | 41.77 (53.2) |
| Candidiasis of the gastrointestinal tract, respiratory organs, genitourinary system | 5.77 (6.5) |
| Kaposi's sarcoma | 1.77 (1.3) |
| Shingles | 3.77(3.9) |
| Encephalitis | 13.77 (16.9) |
| Cryptococcosis | 1.77 (1.3) |
| Cancer | 3.77 (3.9) |
| Lymphoproliferative disease | 1.77 (1.3) |
| Breast cancer | 0.34 (0) |
| Cancer of the vulva | 1.34 (2.9) |
| Kidney cancer | 1.77 (1.3) |
| Body mass index below 10% | 30.77 (39.0) |
| Sepsis | 5.77 (6.5) |
| Bacterial pneumonia | 36.77 (46.8) |
| Fungal pneumonia | 16.77 (20.8) |
| Urinary tract infection | 5.77 (6.5) |
| Associated chronic infectious agents | |
| Chronic viral hepatitis C | 23.76 (29.9) |
| Chronic viral hepatitis C treated | 1.77 (1.3) |
| Chronic viral hepatitis B | 2.77 (2.6) |
| Traditional risk factors for severe COVID-19 | |
| Diabetes | 0.77 (0) |
| Obesity | 2.77 (2.6) |
| Arterial hypertension | 4.77 (5.2) |
| Chronic kidney disease | 3.77 (3.9) |
| Gout | 0.77 (0) |
The Characteristics of Patients with COVID-19 and Previously Known Advanced HIV Disease Who Did Not Choose to Disclose Their HIV Status a
Group 1 experienced a more severe course of COVID-19. Among these patients, CT scans of the chest revealed more extensive lung damage, with a notably higher percentage of individuals exhibiting critical CT-4 lesions (33.1% in group 1 compared to 12% in group 2), as illustrated in Figure 2.
Although not all patients from group 2 were adherent to ART and had an undetectable HIV viral load, in general, patients from group 1 demonstrated more severe immunodeficiency (Figure 3).
We additionally selected a subgroup of patients from group 2 who were adherent to ART and had a suppressed HIV viral load. In this subgroup, the mortality rate was (10.7% CI, 4.0 - 23.5%), as shown in Table 3.
| Variables | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Gender | M | M | M |
| Age | 42 | 56 | 49 |
| Years have passed since the diagnosis of HIV infection | 9 | 20 | 5 |
| Years have passed since the beginning of ART | 2 months | unknown | unknown |
| Immune status | CD4 33 cells/µL, CD8 453 cells/µL, CD4/CD8 0.07 | CD4 151 cells/µL, CD8 153 cells/µL, CD4/CD8 0.98 | CD4 301 cells/µL, CD8 419 cells/µL CD4/CD8 0.71 |
| ART scheme | DRV600/r + 3TC + TDF | RAL + 3TC + RPV | EFV + 3TC + TDF |
| Opportunistic diseases | Pathological anatomical diagnosis: Bilateral polysegmental pneumonia of mixed etiology (intra-vital detection of Herpes simplex virus 2, Klebsiella pneumoniae, Candida albicans, Candida glabrata DNA in sputum; autopsy tank; examination of lung tissue: Growth of Pseudomonas aeruginosa) with abscess formation. Subacute leptomeningitis (autopsy bacterial examination of cerebrospinal fluid: Growth of Klebsiella pneumoniae, Pseudomonas aeruginosa). Kaposi's sarcoma of the skin on the face, trunk, and extremities with metastases to the base of the tongue, larynx, and lungs. Mycotic erosive and ulcerative esophagitis. Cachexia. Primary complications: Respiratory distress syndrome. Inflammatory syndrome associated with immune system restoration. Necronephrosis. Disseminated intravascular coagulation (DIC) syndrome: Hemorrhages in the conjunctiva, subpleural membrane, and epicardium; splenic involvement; blood clots in the vessels of internal organs and the brain; focal necrosis of the pancreas and adrenal glands. Pseudomembranous enterocolitis with predominant involvement of the large intestine (bacterial study of contents from the small and large intestine: Growth of Candida species was detected, analysis for Clostridium was negative). | Pathological anatomical diagnosis: Bilateral polysegmental pneumonia of mixed etiology (microbiological examination of the lungs during autopsy detected Klebsiella pneumoniae and fungus pseudomycelium was detected histobacterioscopically). Primary complication: Diffuse alveolar damage in the exudative phase. Disseminated intravascular coagulation (DIC) syndrome: Widespread hemorrhages, thrombosis of small vessels in the skin, mucous membranes of the respiratory, digestive, and urinary-genital tracts in the lungs, kidneys, and adrenal glands; hemorrhagic erosion of the stomach, focal necrosis of the adrenal glands and pancreas. Acute renal failure: Proteinuria 1.45 g/L; uremia with creatinine level of 314 µmol/L and urea level of 29.3 mmol/L. Coexisting conditions: Obesity. Chronic viral hepatitis C. Underlying disease: Chronic mesangiocapillary glomerulonephritis. Renal arterial hypertension with eccentric myocardial hypertrophy (heart weight 360 g, left ventricular wall thickness 1.7 cm, right ventricle 0.3 cm). | Bilateral polysegmental pneumonia of a severe course. Systemic inflammatory response syndrome of infectious origin with organ dysfunction. Severe anemia. History of Kaposi's sarcoma. |
The Characteristics of Patients Who Were Adherent to Antiretroviral Therapy (ART) and Had a Suppressed HIV Viral Load and Died
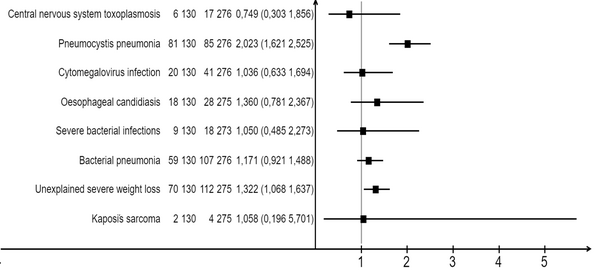
The most significant opportunistic infections and conditions that increased the risk of death in patients with both COVID-19 and newly diagnosed HIV infection were pneumocystis pneumonia and a body weight deficiency of more than 10%. No significant differences were observed between groups 1 and 2 in terms of patients with cytomegalovirus (CMV) pneumonitis, bacterial pneumonia, and sepsis, as indicated in Table 4 and Figure 4.
| Patient Status | N | n | Mortality Rate (%) | Relative Risk | P-Value |
|---|---|---|---|---|---|
| Central nervous system toxoplasmosis | 0.75 | 0.354 | |||
| Newly diagnosed advanced HIV disease | 130 | 6 | 4.6 | ||
| Previously diagnosed advanced HIV disease | 276 | 17 | 6.2 | ||
| Pneumocystis pneumoniaa | 2.02 | < 0.001 | |||
| Newly diagnosed advanced HIV disease | 130 | 81 | 62.3 | ||
| Previously diagnosed advanced HIV disease | 276 | 85 | 30.8 | ||
| Cytomegalovirus infection with lung involvement b | 1.04 | 0.499 | |||
| Newly diagnosed advanced HIV disease | 130 | 20 | 15.4 | ||
| Previously diagnosed advanced HIV disease | 276 | 41 | 14.9 | ||
| Oesophageal candidiasis (or candidiasis of the trachea, bronchi, or lungs) | 1.36 | 0.179 | |||
| Newly diagnosed advanced HIV disease | 130 | 18 | 13.8 | ||
| Previously diagnosed advanced HIV disease | 275 | 28 | 10.2 | ||
| Severe bacterial infections (bacteremia) | 1.05 | 0.527 | |||
| Newly diagnosed advanced HIV disease | 130 | 9 | 6.9 | ||
| Previously diagnosed advanced HIV disease | 273 | 18 | 6.6 | ||
| Bacterial pneumonia | 1.17 | 0.124 | |||
| Newly diagnosed advanced HIV disease | 130 | 59 | 45.4 | ||
| Previously diagnosed advanced HIV disease | 276 | 107 | 38.8 | ||
| Unexplained severe weight loss (> 10% of presumed or measured body weight) | 1.32 | 0.009 | |||
| Newly diagnosed advanced HIV disease | 130 | 70 | 53.8 | ||
| Previously diagnosed advanced HIV disease | 275 | 112 | 40.7 | ||
| Kaposi's sarcoma | 1.06 | 0.636 | |||
| Newly diagnosed advanced HIV disease | 130 | 2 | 1.5 | ||
| Previously diagnosed advanced HIV disease | 275 | 4 | 1.5 |
Factors Affecting the Risk of Death in Patients with COVID-19 and Newly Diagnosed Advanced HIV Disease
5. Discussion
Dramatic differences in COVID-19 outcomes in the group of patients with COVID-19 and newly diagnosed advanced HIV disease at the time of hospitalization and in the patient group with COVID-19 and previously diagnosed advanced HIV disease can be explained by the fact that that there are a number of signs that can mislead clinicians when providing care to patients with COVID-19 and advanced HIV disease.
First, differentiating COVID-19 pneumonia from other lung infections can be very difficult, especially from other viral or atypical pneumonia (9). The spectrum of HIV-associated opportunistic pneumonia is wide and includes bacterial, mycobacterial, fungal, viral, and parasitic pneumonia (10). In patients with immunodeficiency due to HIV infection and COVID-19, it is most often necessary to differentiate lung damage from Pneumocystis pneumonia, cytomegalovirus pneumonitis, fungal infections, and bacterial pneumonia due to the similarity of the CT image (11).
Pneumonia, which is caused by Pneumocystis jirovecii, is characterized by long-term increases in shortness of breath (for weeks or months), fever, and dry cough. Pneumocystis pneumonia is often combined with oropharyngeal candidiasis (12, 13). During a CT examination, the following signs should be taken into account: A “ground glass” pattern, the affected areas are located more often in the root of the lung and the middle zone, thickening of the interlobular septa, which, in combination with ground glass type consolidations, can form a cobblestone pattern pavement (11, 14-19). Air cysts, areas of consolidation, focal compaction, and a complication of pneumocystis pneumonia-spontaneous pneumothorax can also form (14-19). To confirm the diagnosis, the PCR testing of sputum and bronchoalveolar lavage should be used if the patient’s condition is stable (12).
Isolated lung damage during CMV infection develops only with severe immunodeficiency (CD4 + below 50 cells/mm3) (20); however, combined damage with other opportunistic infections can occur with a higher immune status. Clinically, CMV pneumonitis is indistinguishable from pneumocystis pneumonia; however, one should remember the possible multiple organ damage during CMV infection: In addition to pneumonitis, the development of retinitis, encephalitis, ulcerative colitis, esophagitis, pancreatitis, and polyneuropathy is possible (21). The CT scan of the lungs with CMV pneumonitis is similar to the lesions with pneumocystis pneumonia and can be combined; however, there are a number of differences: Ground glass lesions with cytomegalovirus infection appear in the peripheral parts of the lower lobes of the lungs and gradually spread to the roots, and with pneumocystis pneumonia, lesions spread from the roots to the periphery “like the wings of a butterfly” (10, 20). The small focal consolidation characteristics of CMV infection are also relatively uncommon in pneumocystis pneumonia (PCP) (10), as it is a pleural effusion (22). The diagnosis of CMV infection of target organs is usually made based on the clinical picture and, if possible, the presence of the virus in tissues. Histological examination reveals cytomegaly and intracellular inclusions, surrounded by a clear halo in the form of an “owl’s eye” (23). The pp65 antigen test is highly sensitive but cannot be used in patients with leukopenia because this test detects antigens in neutrophils (24). A combination of clinical manifestations, a significant decrease in CD4+ cells per mm3, CT imaging, and PCR analysis of blood, sputum, and bronchoalveolar lavage can also provide valuable information.
Aspergillosis is extremely rare, even in patients with HIV infection (25), but the widespread use of corticosteroids for COVID-19 has led to an increased incidence of Aspergillosis in the general population (26). Risk factors for aspergillosis include neutropenia (absolute neutrophil count less than 1,000) and a history of corticosteroid use (24, 25). Clinical manifestations may vary depending on the form but are generally not specific: Fever, cough, and shortness of breath. Hemoptysis can occur when the cavity is localized in the upper lobes of the lungs or in the obstructive form of bronchopulmonary aspergillosis (25-29). Typical pulmonary CT findings include multiple poorly defined peripheral small lesions, predominantly in the upper lobes of the lungs, which gradually coalesce into larger masses or areas of lobular to diffuse consolidation. These lesions consist of a hemorrhagic component and post-infarction lung tissue. When reabsorption of necrotic tissue begins, this is manifested by the retraction of the infarct zone, and air fills the space between them - the 'air crescent sign' manifests as the presence of a crescent-shaped air pocket surrounding the soft tissue component of the pathological cavity. Additionally, the 'Monod sign' may be observed, wherein the Aspergillus-formed ball within the cavity shifts when the patient's body position changes during a CT examination (10, 11, 16).
The clinical picture of community-acquired pneumonia does not differ from that of patients without HIV, but there is a tendency for rapid progression (30). Typical CT findings include unilateral focal consolidation. Other CT findings include cavity formation, pleural effusion, and lymphadenopathy. Ground glass changes may be present very early in the disease (11). Pleural empyema may be present in pneumonia caused by Staphylococcus aureus (11, 30).
Second, lymphopenia of COVID-19 is also present in HIV infection, especially in the later stages (31). Acute SARS-CoV-2 infection is associated with lymphopenia in approximately 80% of patients (32). This may be due to the production of excessive amounts of pro-inflammatory cytokines due to COVID-19 infection, which induces lymphocyte apoptosis (33-35). There is also evidence that during severe COVID-19, blood lactate levels increase, and this may suppress lymphocyte production (36). As for HIV infection, during its natural course, during the acute phase, there is a sharp decrease in CD4+T lymphocytes, and when infected CD4+T lymphocytes are completely destroyed, they release more copies of HIV into the bloodstream (37). These new copies of the virus find and attack more CD4 cells, and the cycle continues. This results in far fewer HIV-free, functionally active CD4 cells. In the absence of HIV suppression by ART, the lymphocyte population becomes depleted over time and is characterized by a low CD4/CD8 ratio (38).
Third, in patients with severe immunodeficiency, SARS-CoV-2 viral shedding persists for an extremely long time (39, 40) and, having a confirmed diagnosis in hand, the practitioner may not continue the diagnostic search in the area of concomitant infections, which may determine the severity of the patient’s condition.
5.1. Conclusions
The similarity of CT picture, lymphopenia, and prolonged viral shedding of SARS-CoV-2 may mislead doctors who treat patients with COVID-19. We recommend HIV testing in patients with severe COVID-19 to determine appropriate management because mortality in the patient group with COVID-19 and newly diagnosed advanced HIV disease at the time of hospitalization was extremely high and amounted to 46.2% (CI, 38.2 - 54.3%). The presence of this factor in the overall study cohort of patients increased the risk of death by 2.21 times.