1. Background
Dental caries remain a major global health concern, primarily due to biofilm formation by Streptococcus mutans in the oral cavity (1, 2). Streptococcus mutans contributes to tooth decay by converting dietary sugars into acids, leading to enamel demineralization and cavity formation (3, 4). Current preventative measures, such as fluoride treatments and improved oral hygiene, are not always effective in controlling the bacterial colonization responsible for dental caries (5-7). This has led to a growing interest in alternative therapies like photodynamic therapy (PDT) and cold plasma treatment as adjuncts to traditional methods (8-11). Although PDT and cold atmospheric plasma (CAP) have individually demonstrated antimicrobial efficacy (9, 12, 13), there is a distinct lack of studies that directly compare these two modalities under standardized conditions. In particular, comparative data evaluating different PDT wavelengths and photosensitizers alongside CAP exposure durations against S. mutans are scarce. This gap limits our understanding of which parameters may offer the greatest antibacterial effect and hinders translation into clinical protocols.
Chlorhexidine (CHX) is a well-established antimicrobial and the gold standard in dentistry due to its broad-spectrum bactericidal activity (14). However, despite its effectiveness, CHX is associated with several side effects, including staining of teeth and oral tissues, taste alteration, and cytotoxic effects on human cells. These limitations have led to the exploration of newer, non-toxic alternatives that could provide effective bacterial control while minimizing adverse effects (15-17).
Photodynamic therapy is a non-invasive therapy that uses a photosensitizing agent, such as methylene blue (MB) or indocyanine green (ICG), activated by light at specific wavelengths. This activation generates reactive oxygen species (ROS) that damage bacterial cell walls and proteins (18-20). Studies have demonstrated PDT’s potential against dental pathogens, but the effectiveness can vary based on the wavelength used and the specific photosensitizer employed (9). In this study, the 660 nm wavelength was selected for its strong activation of MB, a widely used photosensitizer with proven efficacy against gram-positive bacteria and excellent ROS yield. The 810 nm wavelength was chosen for its compatibility with ICG, which offers deeper tissue penetration and selective bacterial uptake. Both MB and ICG have been well-studied in dental applications, with favorable safety profiles and photophysical properties that make them suitable for antimicrobial PDT (9, 18-20).
Cold atmospheric plasma has emerged as another promising antimicrobial treatment (12). Cold plasma generates a combination of reactive species, UV radiation, and charged particles that can disrupt microbial biofilms without causing significant harm to surrounding tissue (21, 22). While PDT and cold plasma have been investigated individually, direct comparative studies evaluating their efficacy on S. mutans are limited (23-26). Previous research has largely focused on either PDT or plasma as standalone treatments, without examining the potential differences in effectiveness between various wavelengths of light in PDT or comparing them to plasma. For example, studies by Ahrari et al. (27) and Bueno-Silva et al. (28) have explored the use of PDT against S. mutans, but they did not consider different wavelengths within the same experimental framework. Similarly, research on cold plasma has shown significant antimicrobial effects on dental pathogens, but the potential for combining or comparing these modalities remains underexplored.
2. Objectives
Given this gap in the literature, the present study aims to compare the antibacterial effects of PDT at two different wavelengths (810 nm and 660 nm) using two photosensitizers (ICG and MB) with cold plasma treatment and CHX against S. mutans. This comparison will provide insights into the more effective method for reducing bacterial populations, potentially guiding future dental treatments.
3. Methods
3.1. Study Design and Sample Size Determination
This study was designed as an in vitro experimental study. The sample size was determined based on findings from Nima et al. (29) using a one-way ANOVA power analysis conducted in PASS11 software (NCSS, LLC, Utah, USA), which was selected for its reliability and precision in computing power analyses across various experimental designs. The analysis applied an alpha level (α) of 0.05, a beta level (β) of 0.2, an effect size of 1.13, and an estimated standard deviation of 0.48 for the logarithmic colony counts of S. mutans. Results indicated that at least four samples per group across the 13 experimental groups were necessary to ensure sufficient statistical power.
3.2. Sample Preparation
All samples were prepared using the standard strain S. mutans (ATCC str.m 1683), which was acquired from the Pasteur Institute in Tehran. Bacterial samples were diluted in sterile saline, and swabs saturated with S. mutans were inoculated onto petri dishes with 5% sheep blood agar. The cultures were incubated aerobically at 37°C for 48 hours. Streptococcus mutans was then diluted to a 0.5 McFarland standard (approximately 1.5 × 108 bacteria/mL) and dispensed into wells of 96-well microplates (30, 31).
3.3. Experimental Groups
The prepared bacterial samples were divided into 13 experimental groups:
- Group 1 - positive control group: 0.5 McFarland S. mutans suspension with no treatment.
- Group 2 - negative control group: No sample with no treatment.
- Group 3 - ICG group: 0.5 mL of ICG (Sina Pishgam-Darou Co., Iran) at a concentration of 0.2% without laser irradiation (32).
- Group 4 - MB group: 0.5 mL of MB (Merck, Germany) at a concentration of 0.02% without laser irradiation (30).
- Group 5 - 810 nm laser irradiation group: 60-second 810 nm laser irradiation without any photosensitizer.
- Group 6 - 660 nm laser irradiation group: 100-second 660 nm laser irradiation without any photosensitizer.
- Group 7 - 810 nm laser irradiation with ICG group: 0.5 mL of ICG at a concentration of 0.2% with 60-second 810 nm laser irradiation.
- Group 8 - 660 nm laser irradiation with MB group: 0.5 mL of MB at a concentration of 0.02% with 100-second 660 nm laser irradiation.
- Group 9 - 90 seconds CAP treatment.
- Group 10 - 120 seconds CAP treatment.
- Group 11 - 150 seconds CAP treatment.
- Group 12 - 180 seconds CAP treatment.
- Group 13 - CHX group: 0.1 mL of CHX at a concentration of 2% (DarouPakhsh, Iran) was added to the bacterial suspension as the gold standard treatment for S. mutans (14).
3.4. Laser Parameters
In the 810 nm laser groups, a Fox 810 nm laser device (Germany) with a power output of 100 mW and an energy density (ED) of 12 J/cm2 was utilized for 60 seconds. The diameter of the laser tip was 0.8 cm (30). In the 660 nm laser groups, a Sirona 660 nm laser device (Germany) with a power output of 100 mW and an ED of 20 J/cm2 was utilized for 100 seconds. The diameter of the laser tip was 0.8 cm (9). The ED of all laser devices was calculated using the following formula (33):
In all samples, the laser beam was directed tangentially at the openings of the wells.
3.5. Cold Atmospheric Plasma Parameters
In CAP-treated groups, 30 µL of cell suspension was placed into selected wells of 96-well microtiter plates, with plasma treatment lasting for 90, 120, 150, and 180 seconds (34). Plasma treatment was conducted with the PlasmArt device (Nariatech, Iran), which ionized helium gas within a dielectric chamber to produce cold plasma. The device operated at an inlet pressure of 4.5 bar, with a gas flow rate of 1.85 cm3/s, a power output of 8 W, and a handpiece frequency of 100 kHz. The plasma flame measured 21 mm in length and 2.5 mm in diameter, with voltage adjusted up to 10 kV. A manometer monitored the gas output pressure.
3.6. Colony Count
To assess the bactericidal effects of the treatments, 10 µL of untreated and treated bacterial suspensions were inoculated onto separate blood agar plates. The plates were incubated at 37°C for 48 hours to promote colony growth. After incubation, colonies of S. mutans were counted to determine colony-forming units (CFU/mL), providing a measure of bacterial viability post-treatment. Colony counts were conducted using a colony counter, and the results were recorded for subsequent statistical analysis (30, 35).
3.7. Data Analysis
Statistical analyses were conducted using SPSS software version 26. A one-way ANOVA was used to compare the CFU/mL counts across the different experimental groups. Post-hoc comparisons were performed using the Tamhane test when appropriate. A P-value of less than 0.05 was considered statistically significant, indicating a meaningful difference between the treatment groups.
4. Results
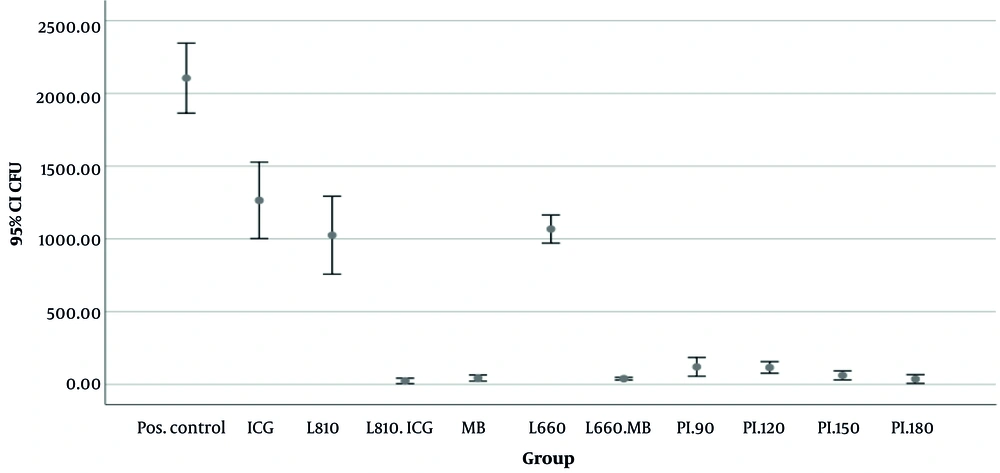
Figure 1 illustrates the mean colony counts across the experimental groups. A statistically significant difference in colony counts was observed among the groups (P < 0.001). The CHX-treated group exhibited the lowest mean colony count (0.00 ± 0.00), followed closely by the group treated with 810 nm laser with ICG (23.5 ± 11.67), 180-second CAP treatment (36.75 ± 18.87), and the group treated with 660 nm laser with MB (39.25 ± 5.62). The highest colony counts were recorded in the positive control group (no treatment; 2105 ± 151.10) and the ICG group without laser irradiation (1264 ± 165.04).
Tables 1 to 3 presents the pairwise comparisons of S. mutans CFU/mL across the treatment groups. Results indicate that all treatment methods significantly reduced colony counts compared to the positive control group (P < 0.05). The CHX-treated group showed a notably lower colony count than other treatments, underscoring its superior efficacy (P < 0.05).
| Groups | P-Value |
|---|---|
| Positive control | |
| ICG | 0.016 a |
| 810 nm laser | 0.004 b |
| 810 nm laser with ICG | 0.005 b |
| MB | 0.005 b |
| 660 nm laser | 0.013 a |
| 660 nm laser with MB | 0.006 b |
| 810 nm laser with ICG | |
| ICG | 0.033 a |
| 810 nm laser | 0.065 |
| MB | 0.979 |
| 660 nm laser | 0.002 b |
| 660 nm laser with MB | 0.978 |
| 90 (s) CAP | 0.541 |
| 120 (s) CAP | 0.109 |
| 150 (s) CAP | 0.676 |
| 180 (s) CAP | 1.000 |
| 660 nm laser with MB | |
| ICG | 0.035 a |
| 810 nm laser | 0.070 |
| MB | 1.000 |
| 660 nm laser | 0.003 b |
| 90 (s) CAP | 0.781 |
| 120 (s) CAP | 0.325 |
| 150 (s) CAP | 0.997 |
| 180 (s) CAP | 1.000 |
| 810 nm laser | |
| ICG | 0.994 |
| MB | 0.068 |
| 660 nm laser | 1.000 |
| 90 (s) CAP | 0.060 |
| 120 (s) CAP | 0.076 |
| 150 (s) CAP | 0.069 |
| 180 (s) CAP | 0.064 |
| 660 nm laser | |
| ICG | 0.995 |
| MB | 0.002 b |
| 90 (s) CAP | 0.000 c |
| 120 (s) CAP | 0.000 c |
| 150 (s) CAP | 0.001 b |
| 180 (s) CAP | 0.001 b |
Laser Groups Pairwise Comparisons and Their P-Values
| Groups | P-Value |
|---|---|
| Positive control | |
| 90 (s) CAP | 0.003 a |
| 120 (s) CAP | 0.005 a |
| 150 (s) CAP | 0.005 a |
| 180 (s) CAP | 0.005 a |
| 180 (s) CAP | |
| ICG | 0.032 b |
| MB | 1.000 |
| 810 nm laser | 0.064 |
| 810 nm laser with ICG | 1.000 |
| 660 nm laser | 0.001 a |
| 660 nm laser with MB | 1.000 |
| 90 (s) CAP | 0.642 |
| 120 (s) CAP | 0.148 |
| 150 (s) CAP | 0.999 |
| 150 (s) CAP | |
| ICG | 0.033 b |
| MB | 1.000 |
| 810 nm laser | 0.069 |
| 810 nm laser with ICG | 0.676 |
| 660 nm laser | 0.001 a |
| 660 nm laser with MB | 0.997 |
| 90 (s) CAP | 0.958 |
| 120 (s) CAP | 0.572 |
| 120 (s) CAP | |
| ICG | 0.035 b |
| MB | 0.232 |
| 810 nm laser | 0.076 |
| 810 nm laser with ICG | 0.109 |
| 660 nm laser | 0.000 c |
| 660 nm laser with MB | 0.325 |
| 90 (s) CAP | 1.000 |
| 90 (s) CAP | |
| ICG | 0.026 b |
| MB | 0.777 |
| 810 nm laser | 0.060 |
| 810 nm laser with ICG | 0.541 |
| 660 nm laser | 0.000 c |
| 660 nm laser with MB | 0.781 |
Cold Plasma Groups Pairwise Comparisons and Their P-Values
| Groups | P-Value |
|---|---|
| CHX | |
| Positive control | 0.000 a |
| ICG | 0.001 b |
| 810 nm laser | 0.001 b |
| 810 nm laser with ICG | 0.028 c |
| MB | 0.007 b |
| 660 nm laser | 0.000 a |
| 660 nm laser with MB | 0.001 b |
| 90 (s) CAP | 0.010 c |
| 120 (s) CAP | 0.003 b |
| 150 (s) CAP | 0.008 b |
| 180 (s) CAP | 0.030 c |
| 810 nm laser | 0.070 |
| MB | 1.000 |
| 660 nm laser | 0.003 b |
| 90 (s) CAP | 0.781 |
| 120 (s) CAP | 0.325 |
| 150 (s) CAP | 0.997 |
Chlorhexidine Group Pairwise Comparisons and Their P-Values
In addition, the results indicated that, regardless of the exposure time in the CAP-treated groups or the type of photosensitizer used (either ICG or MB) in the laser-treated groups, all CAP-treated groups and laser irradiations combined with photosensitizers resulted in significantly lower colony counts compared to the 660 nm laser irradiation alone or ICG alone (P < 0.05). When comparing different laser irradiation groups, no statistically significant difference was found between the 660 nm and 810 nm lasers when photosensitizers were not used (P > 0.05). Similarly, no statistically significant differences were found between CAP exposure times, although the 180-second exposure resulted in lower mean colony counts compared to the 150-, 120-, and 90-second exposures (36.75 ± 18.87, 61.50 ± 19.29, 115.75 ± 25.01, and 120 ± 40.62, respectively). When photosensitizer was applied alone, MB showed a significantly lower colony count than ICG (mean colony count, MB = 43.25 ± 13.27, ICG = 1264.5 ± 165.04, and P = 0.03).
5. Discussion
This study evaluated the antibacterial efficacy of PDT with 810 nm and 660 nm lasers, CAP treatment at varying exposure times, and CHX against S. mutans colonies. Results revealed significant bacterial reduction across all treatments compared to the control group, with CHX achieving complete eradication of colonies, followed by PDT with the 810 nm laser combined with ICG as a photosensitizer, CAP at 180 seconds, and PDT with the 660 nm laser combined with MB as a photosensitizer. Each treatment’s unique mechanism of action contributes to its respective antibacterial efficacy, underscoring their potential as an alternative or adjunctive antimicrobial treatment in clinical applications.
Chlorhexidine displayed the highest antibacterial efficacy, resulting in zero detectable mean colony counts. Known for its rapid and broad-spectrum action, CHX disrupts bacterial cell membranes and binds strongly to negatively charged cell surfaces, leading to cell death (36-38). These characteristics make it the gold standard in dental antimicrobial treatments, particularly effective in biofilm-associated infections (14-16, 39). However, CHX’s side effects, including tooth staining, taste alteration, and cytotoxicity, present limitations in long-term or widespread use, underscoring the need for alternative therapies (15, 39).
In this study, PDT with the 810 nm laser and ICG demonstrated high bactericidal activity, reducing S. mutans colony counts to levels comparable to CHX. The effectiveness of PDT with ICG under the 810 nm wavelength can be attributed to enhanced ROS generation, which is critical for bacterial inactivation (40). Activated by the 810 nm laser, ICG produces singlet oxygen and other ROS that cause extensive damage to bacterial cell membranes and intracellular structures. The deep penetration of the 810 nm wavelength enables the laser to target bacteria within deeper tissue layers, while ICG’s hydrophilic properties facilitate its binding to bacterial cells, ensuring selective targeting with minimal collateral damage (40, 41). These properties position 810 nm PDT with ICG as a promising, targeted approach for clinical use.
Our study found that ICG alone was ineffective in reducing S. mutans colonies, consistent with existing literature that emphasizes ICG's reliance on light activation to produce ROS for antimicrobial action (40, 41). Studies, such as by Kim et al. (32), confirm that ICG requires specific laser activation, like the 810 nm wavelength, to achieve bactericidal effects, particularly against biofilms. Without this activation, ICG lacks intrinsic antibacterial properties, underscoring the importance of using it in combination with appropriate laser irradiation for effective antimicrobial treatment (40-42).
Among CAP-treated groups, the 180-second exposure produced the lowest colony counts, illustrating the advantages of longer CAP treatment durations. CAP operates by generating ROS, reactive nitrogen species (RNS), and UV photons, which act synergistically to cause oxidative stress, disrupt cell membranes, and damage bacterial DNA (12, 19, 21). Longer exposure times allow for a sustained release and accumulation of these reactive species, intensifying oxidative damage to bacterial cells. This prolonged interaction increases ROS and RNS concentrations around bacterial cells, overwhelming bacterial defenses and ensuring a more comprehensive inactivation (19).
Extended CAP exposure times are particularly beneficial for biofilm-associated bacteria (43). In biofilms, bacteria are encased within an extracellular matrix, which can impede the penetration of reactive species during shorter treatments. The sustained action from longer CAP exposure overcomes this barrier, allowing ROS and RNS to penetrate deeper into biofilm layers and reach bacteria that might evade shorter treatments (19, 43). Additionally, prolonged exposure reduces the potential for bacterial recovery or resistance by applying continuous oxidative stress, exhausting bacterial repair mechanisms and leading to more extensive cell death. This sustained action makes CAP, especially with extended durations, a powerful option for treating biofilm-related infections (12, 19, 21, 43).
Similar to the results of our study, Suhail Ali et al. (44) demonstrated CAP’s efficacy in reducing S. mutans biofilms, emphasizing its biofilm penetration ability and robust bactericidal effects, especially with longer exposure times. Similarly, Figueira et al. found that CAP treatments could significantly reduce bacterial loads across a range of pathogens, with effects comparable to traditional antimicrobials in some cases (25, 45).
The findings demonstrated that the 660 nm laser combined with MB had significantly greater bactericidal efficacy than ICG. Methylene blue’s superior performance can be attributed to its higher ROS yield when activated by 660 nm light and its strong binding affinity for bacterial cell membranes (35). As a positively charged molecule, MB effectively adheres to the negatively charged bacterial cell surface, resulting in targeted ROS generation that disrupts membrane integrity, proteins, and DNA. Methylene blue’s stability under light exposure further ensures a sustained bactericidal effect throughout the PDT session, enhancing its efficacy against S. mutans. These properties underscore MB’s potential as a highly effective photosensitizer in PDT applications, particularly in low-oxygen environments, such as biofilms, where anaerobic pathogens thrive (35, 46).
The 660 nm laser without a photosensitizer produced significantly higher colony counts than PDT and CAP treatments, underscoring the limited efficacy of laser irradiation alone. Without a photosensitizer to generate ROS, the 660 nm laser lacks the primary mechanism for bacterial inactivation, resulting in minimal impact on colony counts (9, 35, 47). Moreover, the power level of the 660 nm laser in this study might be insufficient to induce a thermal effect capable of disrupting bacterial cells, reinforcing the need for photosensitizers like MB or ICG in PDT applications (9, 18).
While PDT and CAP demonstrated strong antibacterial effects in this study, several practical challenges may limit their widespread clinical implementation. These include high initial equipment costs, the need for specific consumables (e.g., lasers, photosensitizers, plasma devices), and ongoing maintenance expenses. Moreover, access to such technologies is limited in many dental clinics, especially in resource-constrained settings (48). Unlike CHX, which is inexpensive, readily available, and simple to use without specialized training, both PDT and CAP require operator expertise, treatment planning, and careful parameter control. Additionally, limited clinician training and the absence of standardized protocols or strong clinical guideline endorsements may hinder adoption (17, 18, 21, 48). These factors should be carefully considered when evaluating the feasibility of integrating PDT and CAP into routine dental care, especially when compared to the established practicality of CHX.
While this study provides valuable insights into the bactericidal effects of PDT, CAP, and CHX, certain limitations should be acknowledged. The study was conducted in vitro, and as with all in vitro studies, there are inherent limitations related to the controlled laboratory environment that may introduce bias. The absence of host factors such as saliva flow, immune responses, and oral microbiome interactions may over- or under-estimate the true efficacy of PDT and CAP in vivo. Furthermore, biofilm behavior and treatment diffusion in clinical conditions may differ significantly from static in vitro setups. These factors should be considered when interpreting the results and planning for future in vivo studies. Additionally, the study focused on S. mutans, which, while relevant, may not reflect the responses of other clinically relevant bacteria. Incorporating a broader range of bacterial species and biofilm models would provide a more comprehensive understanding of these treatments. Furthermore, while standardized parameters were used for CAP and laser treatments, variations in CAP gas type, exposure times, or laser power levels may yield different results, warranting additional dose-response studies to optimize treatment efficacy.
5.1. Conclusions
In conclusion, while CHX remains the gold standard due to its exceptional bactericidal properties, PDT and CAP represent valuable additions to antimicrobial treatment options. The unique antibacterial mechanisms and respective strengths of each modality provide clinicians with flexible, effective tools for managing a variety of bacterial infections. Future research, including in vivo studies, optimization of photosensitizer concentrations, and fine-tuning CAP exposure times, will be crucial to fully realizing the clinical potential of PDT and CAP as safe, effective antimicrobial therapies.